
3-Glycidyloxypropylmethyldimethoxysilane came into focus during the surge of synthetic polymer science in the mid-to-late twentieth century. As industries looked for ways to bond organic materials with inorganic substances, silane coupling agents started to shape new manufacturing standards. The discovery of glycidyloxy functional groups allowed chemicals like this silane to connect glass, metal, and organic resins in ways that earlier compounds couldn't manage. Early adoption took off in composite material fabrication, where reliability meant the difference between success and project failure. Over the decades, refinements in synthesis and purification standards pushed this silane from a specialty product to a critical building block across several technology frontiers.
3-Glycidyloxypropylmethyldimethoxysilane stands out as an organosilane that features both glycidyl and methyldimethoxysilane groups. This arrangement lets it bridge the divide between hydrophobic and hydrophilic substances, which makes it a go-to surface modifier for everything from electronics to coatings. Companies seeking to improve the performance of adhesives, sealants, and composite interfaces repeatedly turn to this molecule to solve very practical bonding problems that result from differences in thermal expansion, moisture, and chemical exposure. Formulators prize its straightforward addition and reactivity in current production environments.
This silane clocks in with a molecular formula of C10H22O4Si and a typical molecular weight of about 250 grams per mole. In practice, most batches turn up as a clear to slightly yellowish liquid, with a faint but noticeable smell. It sports a boiling point near 290°C and a low viscosity, which makes it easy to blend with solvents or directly with many uncured resins. The epoxy (glycidyl) group brings strong reactivity, letting it link with nucleophilic sites present in a variety of polymers and inorganic surfaces. Sensitivity to moisture needs to be managed, since the methoxysilane portion starts to hydrolyze in water, creating silanols that can go on to bond with glass or other silicate surfaces.
Manufacturers supply 3-glycidyloxypropylmethyldimethoxysilane in high purity, often specifying content above 97% to meet the demands of electronics and aerospace applications. Labels highlight CAS number 65799-47-5, hazardous material codes, and directions for safe storage—typically in dry, cool, and tightly sealed containers to discourage premature hydrolysis. Containers vary from HDPE drums for bulk to glass bottles for lab use. Documentation always includes certificates of analysis, MSDS, and details about stability under storage and processing temperatures.
Typical industrial processes rely on the hydrosilylation route. Producers first react allyl glycidyl ether with methyldimethoxysilane, using platinum-based catalysts to spur addition across the olefin bond without unwanted side reactions. Meticulous temperature control and purification deliver a product that contains minimal residual catalyst and unreacted starting materials. Distillation and controlled hydrolysis cycles maximize yield and purity while maintaining the all-important epoxy functionality. Each preparation run carries the potential for innovation in waste minimization and solvent recovery, which has gained more attention as sustainability pressures mount.
This molecule participates in two big classes of reaction. The glycidyl group opens up to react with nucleophiles like amines, acids, and alcohols, which is why you spot this silane in many adhesives, paints, and coatings. The methyldimethoxysilane portion hydrolyzes to form silanol groups, which then consume moisture to build siloxane networks on surfaces like silica, glass, or ceramics. Combinations allow manufacturers to tailor both bonding and bulk mechanical properties in composite laminates. Research teams also keep tweaking the structure, swapping out substitutes on the silicon or propyl backbone to dial in reactivity, durability, or cure profiles.
This silane wears several names. 3-Glycidyloxypropylmethyldimethoxysilane appears in technical documents. You’ll also see it as Glycidyloxypropylmethyldimethoxysilane, 3-Glycidyloxypropylmethyl-dimethoxysilane, and sometimes by its trade name, KH-570M. Producers and distributors sometimes coin proprietary names or label it as an epoxy silane or a silane coupling agent for simplicity. The CAS registry number—65799-47-5—does the heavy lifting for precise identification across languages and markets.
Anyone handling this silane needs to respect its reactivity and volatility. Standard safety practices call for goggles, gloves, and good ventilation. Contact with eyes or skin can trigger irritation, and inhalation of vapors during large-scale use should be avoided through local exhaust or respiratory protection. Spillage clean-up must target containment and deactivation—water and caustic solutions can neutralize minor leaks. Plant-level hazard analysis and worker training lower accident rates. The chemical asks for careful storage to keep out moisture; once bottles or drums open, any absorbed water will start the hydrolysis reaction, potentially rendering unused product less effective or stable.
This silane finds steady demand in fiberglass-reinforced plastics, epoxy adhesives, paints, and sealants. It’s also critical in semiconductor overcoats and microelectronics assembly, where surface adhesion can impact yield and device reliability. Wind turbine blades, printed circuit boards, and corrosion-resistant coatings draw on its ability to lock together very different kinds of materials. In laboratory R&D, it lets scientists create new organic-inorganic hybrids or modify silica gels to better suit chromatography and separation science. I’ve worked in labs where this material changed the outcome of a stubborn composite formulation; switching to this silane unlocked both processability and toughened the final part.
Research groups stay busy probing ways to improve this silane. In the last ten years, people have begun testing how different catalyst systems, greener solvent choices, and modified reaction conditions can change product yield and limit environmental impact. End-use R&D often centers on durability in automotive or aerospace composites under harsh environments. Analytical chemistry keeps refining detection limits for trace impurities, since those can gum up downstream electronics or optics applications. The latest journals show interest in modifying the propyl chain or silicon substituents for next-generation bonding between nanocomposites and biomaterials.
Toxicology teams have put 3-Glycidyloxypropylmethyldimethoxysilane through a battery of standard safety tests. Results show that, handled with respect, it does not cause acute toxicity at levels typical for industrial or lab settings. Even so, skin sensitization and mild eye irritation can happen, so exposure controls stay important across all stages—development, dye-house, and production. Long-term toxicity and environmental breakdown keep getting studied as governments watch the fate of silicon- and epoxy-bearing compounds in surface waters and waste streams. Regulatory agencies request thorough risk assessments and demand compliance with stricter labeling and reporting standards, especially for uses involving consumer products or medical devices.
As green chemistry keeps gathering steam, future production will focus on making this silane with fewer emissions and better recyclability. R&D aims for less waste, more recoverable catalysts, and bio-based raw materials where possible. On the application front, increasing use in advanced composites, smart surface coatings, and flexible electronics points to continued demand. Work on boosting compatibility with biopolymers and new classes of semiconductors could reveal new market niches. Competition from newer coupling agents with tuned reactivity may nibble at older formulations, yet reliability and proven performance speak volumes in conservative industries. Open collaboration between manufacturers, researchers, and end users will help direct this molecule's future into safer, higher performance, and more responsible hands.
On any regular day, people don’t think about what keeps their countertops sleek, circuit boards running, and paint from flaking away. Chemicals like 3-Glycidyloxypropylmethyldimethoxysilane do tough jobs behind the scenes. I once spent a long afternoon in a materials lab, watching a chemist blend resin with various additives. Even tiny changes made huge differences—edges stopped chipping, adhesives wouldn’t peel. One key tweak was the addition of this odd-named silane.
You see this silane in action across industries. It takes tough jobs in adhesives, helping glues last longer and avoid failures under stress. Glues and sealants often struggle to bond with glass, metal, or even tricky plastics. Regular adhesives might peel off in days or leave gaps you can spot with a magnifying glass. By adding 3-Glycidyloxypropylmethyldimethoxysilane, the adhesion jumps up. That comes from its special structure—one end grabs surfaces, the other bonds with resin or polymers. This means car windshields stay put during hard stops, and electronics don’t shake apart from vibration.
Durability matters whether you're talking about bridges or bathroom tile grout. Over the years, paint and coatings—especially ones used on old buildings and new towers—build up resistance to scrapes and moisture by using silanes. I remember painting a garage floor many summers ago, only to watch it peel before winter’s end. Commercial epoxy floors use the silane for a reason: it keeps out water, resists chemicals, and survives temperature swings. There’s science to it, sure—the silane forms chemical bridges between concrete and resin. End result? Surfaces stay protected, way past a single season.
Anyone who’s cracked open a smartphone sees a packed circuit board—dozens of tiny bits connected and crammed together. Failures often start at the micro-level, where parts separate under heat or vibration. Manufacturers use 3-Glycidyloxypropylmethyldimethoxysilane as a coupling agent. It helps coating materials cling to fragile wafers and wires. The silane cuts the risk of failure during soldering and daily use. Devices keep working, and consumers avoid the frustration of early breakdowns.
A growing number of studies and reports stress the health and safety sides of chemicals like this one. Skin contact, fumes, and long-term exposures can cause real harm in factory settings. I’ve worked near warehouses where safety signage covered entire walls. Gloves, masks, and ventilation weren’t “extra”—they kept skilled workers healthy and on the job. Responsible companies track exposure, follow strong protocols, and push for safer substitutes if health risks grow too high.
Better adhesives, tougher coatings, cleaner electronics—the value of this silane pops up across industries. At the same time, safety questions and environmental risks shouldn’t ever get swept aside. Alternatives pop up as green chemistry advances. In my experience, open discussion and real transparency build trust with customers and workers alike. It works best to keep a close watch on harm while using the strengths of chemistry to make products last longer, perform better, and stay safer.
Unpacking barrels or totes stamped with “3-Glycidyloxypropylmethyldimethoxysilane” sparks memories from time in specialty coatings and adhesives, where this compound finds a use. The name drags, but storage details can’t be shrugged off. I’ve watched carelessness lead to chemical degradation, sticky leaks, and in one ugly memory, singed hands. There’s a reason for precautions: This silane reacts easily with water, can make flammable methanol, irritate skin, and give off fumes that leave you coughing through your mask. Proper storage makes the difference between smooth business and trouble.
Moisture acts almost like a magnet for trouble here. Even small spills in a humid storeroom hydrolyze and kick methanol into the air. One summer, we lost a full drum because someone stored it in a half-open warehouse. The result? Sticky mess, ruined product, and at least a day’s work thrown away. Sealing everything tight is non-negotiable. Tight caps, dry secondary containment, clear “KEEP DRY” labels matter far more than they feel. Desiccant packs land inside smaller containers for another layer of defense.
High temperatures change the game by weakening containers and vaporizing the contents. Low temps sometimes cause crystallization but don’t ruin the material outright. In my experience, room temperature around 20-25°C keeps things steady, though air conditioning isn’t always an option in old warehouses. The point is consistency, not just “cool and dry.” Drastic swings speed up degradation and create pressure build-up, which makes opening a drum a dicey affair. Thermal insulation or shaded, indoor storage does the trick on most sites.
Inhalation hazards rise once vapors start collecting in corners. My old plant had a single fan in a 40-foot storage room, thinking that was enough. Only after maintenance beefed up the vent system – with a real air exchange setup – did headaches among handlers drop. Anyone stashing this silane should consider mechanical ventilation, not just windows propped open. The methanol released is flammable, putting nearby ignition sources such as forklifts or bare-bulb lamps out of the question. Spark-proof switches and grounded containers cut down the fright factor.
Storage next to acids or water-based materials courts disaster. One supervisor, new on the job, placed oxidizers and this silane together for easy restocking. The response team caught it before it went bad, but it could have ended with emergency clean-up and injury. Even in cramped spaces, separating incompatible chemicals each in their own secure area makes sense. Color-coded shelves and chemical logs keep it simple for newcomers and seasoned hands alike.
No system beats old-fashioned training. I saw turnover bring new faces who never handled reactive silanes before. One practical demo on sealed, labeled, sharply segregated storage – plus warning stories from the floor – got everyone on the same page. Material Safety Data Sheets sat in easy reach, and regular walk-throughs caught leaks early. A trained crew learns to spot swelling drums, discolored containers, or sticky residue – and knows to act fast. This isn’t just policy, it’s the reason we all went home without burns or coughs.
Safe storage for specialty chemicals isn’t glamorous, and rarely makes headlines. Yet the quiet effort to get it right protects workers, customers, and the investment itself. No shortcut delivers the same peace of mind as tight seals, dry rooms, and a sharp-eyed crew.Workplaces that handle chemicals face a lot of pressure to keep everyone safe. 3-Glycidyloxypropylmethyldimethoxysilane pops up in coatings, adhesives, electronics, and even the making of plastics. Its full name stumps most people, but workers in labs or factories recognize it because they see it on supply lists and safety sheets. Folks want to know if it is hazardous, toxic, or just tough to pronounce.
Looking at material safety data sheets, this silane compound is considered harmful, especially if you breathe in vapors or let it touch your skin for too long. Eyes are especially sensitive. Inhaling mists can irritate your nose and throat. Getting some on your hands over and over leads to dryness or even dermatitis. A study by the European Chemicals Agency rates it as causing serious eye damage and says not to let the stuff touch your skin without the right gloves. Tests in lab animals show it does not cause cancer in reasonable exposures, but it is linked to moderate toxicity if swallowed, and it triggers chemical burns and allergic skin reactions.
After working with paints and adhesives, the smell of some of these chemicals stays on your hands until the next day. Some coworkers would come away with red spots or itchiness if they skipped gloves. It’s a reminder that just because there’s no skull and crossbones sticker, things can get rough with enough exposure or carelessness.
No one regulates 3-Glycidyloxypropylmethyldimethoxysilane the same way as lead or formaldehyde. Producers must label it as hazardous, but in many countries, small companies sometimes skip the right training. Good ventilation, proper respirators, goggles, and gloves keep most risks at bay. There are fines or warnings for firms that ignore this, but enforcement often depends on inspections and local budgets. You see this in any field, not just chemistry.
Too many people trust instinct. They take off gloves to save time, wear cheap cloth masks, or ignore the pungent fumes. One jobsite handed out material safety data sheets that nobody bothered to read past the first page. After one guy ended up with a nasty eye injury, the boss brought in a real demonstration with proper PPE. That made a difference.
Better training solves a lot. Supervisors should not just hand out MSDS printouts—demonstrating the right way to work with reactive silanes makes abstract risks real. Regular ventilation checks never hurt. Some places update their PPE lists after an accident—smart teams act before that. Suppliers and employers must remember that what looks fine on a shelf can still do real harm if handled wrong.
Using personal experience and proven safety data helps people treat 3-Glycidyloxypropylmethyldimethoxysilane with respect. Industry professionals and regulators have the facts, but safety habits start in the break room and workshop.
3-Glycidyloxypropylmethyldimethoxysilane doesn’t just roll off the tongue and doesn’t play nice without care. This chemical plays an important role in coatings, adhesives, and sealants, where it helps surfaces grab onto each other. It builds lasting bonds between resin and inorganic materials, which turns out to be big business for manufacturers and builders. Ethylene oxide, epoxy groups, and reactive silanes sit together in this molecule, making it useful and pretty lively at the same time.
Spending years in labs and on shop floors, one lesson hits home fast—gloves, goggles, and fresh air matter more than any printed poster on the wall. The safety data sheet urges protection for a reason: direct contact burns skin and eyes, and you can’t tough it out when working with volatile silanes. Respiratory protection isn’t an afterthought either, especially in places without good ventilation. Vapors irritate the lungs and nose. A decent exhaust fan, chemical-resistant gloves (nitrile or butyl rubber), and wraparound goggles should become second nature anytime this chemical comes out of the bottle.
It’s easy to underestimate something clear and runny. One careless move, a small splash or unnoticed drip, and trouble follows. I’ve seen careful operators insist on double-checking lids after dispensing, and experienced teams keep cleanup kits close. Catch spills quickly by relying on absorption pads, and never let the liquid reach drains or soil. Personally, I’ll always remember that one time a careless pour cost half a shift for extra decontamination.
Getting the most out of this silane means respecting a few rules. Dry the surfaces that need treatment. Moisture triggers the silane’s hydrolysis and leads to poor performance, which isn’t just a quality problem but a waste of costly material. A slight excess may help react with surface hydroxyl groups, but adding too much puts future users at risk and introduces unnecessary hazards.
Stirring thoroughly and applying an even coat sounds simple, but shortcuts show up later as peeling, weak adhesion, or tacky surfaces. In big industries, automated spray or dip processes cut down on human error, but plenty of small shops still rely on hand tools and instinct. I’ve learned the value of using dedicated brushes and containers: cross-contamination changes how the silane acts, and nothing ruins a day like finding out the hard way.
Companies pay attention to hazardous waste disposal because both laws and communities demand it. Leftover silane and contaminated personal protective equipment must go in labeled bins for specialized disposal, not the regular trash. Regulations in the EU and US both treat this compound as a hazardous material—there’s no shortcut around records, labeling, and secure storage.
Me, I trust repetitive practice over risky innovation. Training once isn’t enough; hands-on refreshers keep the team sharp. The right culture grows out of people who watch each other’s backs, share tips, and never shrug off a potential shortcut that could cost health or future work.
Experience shows that mindful handling, routine protective gear, and respect for small checks deliver performance and safety together. The chemical itself hardly changes. Results follow people who pay attention, take care, and go back to the basics every time. The stakes sit higher than just product quality—health and business hang in the balance.
Most people outside the specialty chemicals world probably haven’t heard of 3-Glycidyloxypropylmethyldimethoxysilane. It doesn’t roll off the tongue, and you won’t spot it on a supermarket shelf. Still, this compound quietly powers big chunks of the global manufacturing engine. Years spent working in an automotive plant taught me to respect the materials that make tough jobs possible—and this silane is one of them.
Automakers need stronger adhesion between plastics, composites, and metals, especially as cars drop weight to meet fuel standards. 3-Glycidyloxypropylmethyldimethoxysilane steps into that mix. It bonds plastics to metals inside car bodies and dashboards, reducing rattle and wear. My old crew swore by epoxy adhesives with this silane when we switched to more composite car doors. That connection held tight, even on rough roads and wild Michigan temperature swings.
Phones, laptops, and circuit boards live rough lives, soaking up moisture, heat, and fatigue every day. Manufacturers use this silane to coat or treat glass fibers and silicon chips, giving them a barrier that resists cracking and electrical failure. Printed circuit board lines don’t shut down for a bad solder joint—the tolerance for product failure dropped as gadgets became household staples. That’s where the added strength and water-resistance from this silane comes in handy.
Modern construction leans on composite materials—think glass-fiber-reinforced concrete and high-performance sealants. Back in the day, pours cracked, facades chipped off, and nobody wanted to pay for repairs. This silane chemically locks together glass, sand, and resins to cut down on crumbling and water seepage. On the job site, easier workability and longer-lasting performance save time for workers and costs for developers. Keeping rain out and strength up, especially on large projects, has made it a favorite among material engineers.
My uncle ran a small sign-painting business. He loved the glossy finishes that stood up to weather and never peeled. Modern coatings and inks often owe their staying power to tough silanes keeping pigments and binders gripped tightly to glass, metal, or even plastic. In anti-corrosion paints and powder coatings, the silane acts like a double-sided tape at a molecular level, holding everything together. This results in surfaces that look fresh years after application, even in the face of harsh sun, rain, and freezing cold.
Rising environmental pressure keeps pushing industries to phase out harmful chemicals. People want parts that last but also materials that break down clean or recycle easily. To answer that, chemists have started tweaking old formulas, cutting out solvents and maximizing effectiveness. If more manufacturers take up silanes like this one, parts could last longer, machines might run cooler, and we’ll throw away less—small wins, but real ones for heavy industries and the environment alike.
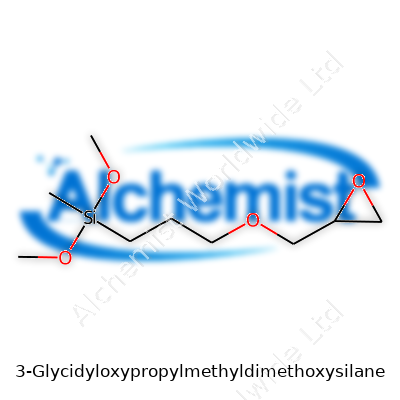
| Names | |
| Preferred IUPAC name | 3-(Oxiran-2-ylmethoxy)propyl(methyl)dimethoxysilane |
| Other names |
Gamma-Glycidoxypropylmethyldimethoxysilane 3-(Glycidyloxy)propylmethyldimethoxysilane Silane, (3-(glycidyloxy)propyl)methyldimethoxy- 3-Glycidyloxypropylmethyldimethoxysilane |
| Pronunciation | /θriː-ɡlɪˌsɪd.ɪ.lɒk.siˌproʊ.pɪlˌmɛθ.əlˌdaɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | [65799-47-5] |
| Beilstein Reference | 805873 |
| ChEBI | CHEBI:82763 |
| ChEMBL | CHEMBL74430 |
| ChemSpider | 157476 |
| DrugBank | DB11206 |
| ECHA InfoCard | 03a30217-efa2-42fd-93df-83a9e32959a1 |
| EC Number | 2530-83-8 |
| Gmelin Reference | 104159. |
| KEGG | C19769 |
| MeSH | D013085 |
| PubChem CID | 66241 |
| RTECS number | VV8400000 |
| UNII | 0LRK2R6ULN |
| UN number | 2735 |
| CompTox Dashboard (EPA) | DTXSID5031322 |
| Properties | |
| Chemical formula | C9H20O4Si |
| Molar mass | 248.36 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.07 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | 0.5 |
| Vapor pressure | 0.13 mmHg (25 °C) |
| Acidity (pKa) | 13.5 |
| Basicity (pKb) | Basicity (pKb): 4.7 |
| Refractive index (nD) | 1.426 |
| Viscosity | 6 cP |
| Dipole moment | 2.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 568.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause an allergic skin reaction. Harmful to aquatic life with long lasting effects. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H317 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P308+P313, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: 126°C |
| Autoignition temperature | 250 °C (482 °F) |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, Rat: 8025 mg/kg |
| NIOSH | GF9840000 |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 200 - < 1000 |
| Related compounds | |
| Related compounds |
3-Glycidyloxypropyltrimethoxysilane 3-Glycidyloxypropyltriethoxysilane 3-Glycidyloxypropylmethyldiethoxysilane 3-Glycidyloxypropylmethyldiethoxysilane hydrochloride 3-(2,3-Epoxypropoxy)propyltrimethoxysilane |