
Chemistry now and then manages to pull the rug out from under our feet. Early surface treatments didn't use the specialized molecular bridges of today. The arrival of 3-Methacryloxypropyltrimethoxysilane—known in the lab as MPTMS and recognized by old-timers as a workhorse organosilane—carved out a new chapter in adhesive chemistry in the 1960s. Researchers grew restless with materials that refused to bond glass to plastic for long without peeling. This molecule’s popularity rode the wave of glass fiber-reinforced plastics through the late 20th century, and it never lost relevance. The drive to make composites stronger and more durable led to widespread adoption in both scientific and industrial circles, with production steadily growing as new manufacturing and coating technologies demanded better interfacial adhesion.
You’ll find 3-Methacryloxypropyltrimethoxysilane in liquid form, usually clear and colorless under lab lights. Its most recognizable feature is a double personality: one end latches on to glass, stone, or metal, while the other loves to link up with polymer chains during curing reactions. This makes it a connector; it’s rarely used on its own but always bumps up the performance of adhesives, sealants, coatings, and composite resins. Manufacturers typically sell this silane by the liter and gallon, labeled with batch-specific purity and water content. Storage calls for dark, dry environments—humidity access leads to hydrolysis and short shelf life.
This silane doesn’t smell strong, but hydrolysis changes can sneak up on sensitive noses. Its boiling point hovers around 290°C, and it starts breaking down if exposed to strong acids or bases at higher temperatures. It weighs in with a molecular weight near 248.3 g/mol and comes with the ability to dissolve in organic solvents like ethanol and acetone, though water triggers a reaction instead of simple dissolution. The methacryloxy group adds chemical reactivity, so handling it in open air means dodging unwanted side-reactions. Density checks in at about 1.045 g/cm³, while its refractive index is often measured at 1.428—both checked in standard supplier certificates, ensuring users are getting the same material batch to batch.
Quality is everything for industrial buyers and university research labs. Suppliers label each drum or bottle with the product code, batch number, and technical grade, usually above 97% purity. Labels warn about moisture, highlight the need for gloves and goggles, and direct users to a detailed Safety Data Sheet (SDS). Specifications go beyond purity—buying agents ask for limits on chlorides, acidity, and color (max 30 APHA, which is nearly water-clear). Methods like gas chromatography and NMR back up the claims on the label, and big buyers expect COA (certificate of analysis) documentation for regulatory audits.
Industrial processes churn out MPTMS mainly through hydrosilylation. Silanol groups are joined to a methacryloxypropyl structure over platinum catalysts under controlled conditions. Workers keep the air dry and watch for exotherms, letting precision equipment mix, heat, and separate by-products. Once synthesis wraps up, purification includes distillation under vacuum to remove volatile remnants. The process demands a sharp eye for moisture control; stray water leads to premature hydrolysis, making the final silane unusable in many applications. Experience matters—production techs check every batch for consistency because even a minor impurity can lead to catastrophic failures in composite strength.
In action, 3-Methacryloxypropyltrimethoxysilane typifies versatility. In water, the methoxy groups swap for silanols, giving way to hydrogen bond formation with silica or glass. The methacrylate tail, meanwhile, copolymerizes readily with unsaturated polyester or acrylics under the influence of free-radical initiators. Chemists sometimes modify the molecule itself or use it as a parent structure for new silanes—optimizing end-use properties like flexibility, hydrophobicity, or adhesion. In research settings, the molecule forms the backbone for studies in controlled surface grafting and functionalized nanoscale coatings, going well beyond simple adhesives.
The name can get tongue-twisting—3-(Trimethoxysilyl)propyl methacrylate shows up on datasheets too. The shorthand “MPTMS” is common. In North American markets, you find offerings under commercial names like Silquest A-174 or Z-6030, depending on the manufacturer. Researchers refer to it as TMSPMA in some publications or write “methacryloxy-functional silane”. A dizzying array of CAS numbers, but the standard is 2530-85-0; accuracy here prevents costly mistakes in purchasing or regulatory filings. Double-checking the label avoids confusion with similar silanes, which may lack the methacryloxy or have different alkoxy tails.
Nothing in the lab or plant moves without safety compliance. Anyone using MPTMS outfits themselves with gloves, goggles, and sometimes face shields, especially if mixing at scale. The molecule irritates eyes, and inhaling vapors might cause respiratory discomfort. Guidelines from OSHA and EU REACH shape local training programs, requiring fume hoods and airtight storage. Emergency protocols call for immediate washing if skin or eyes meet raw material, and environmental controls minimize runoff during production. Safe handling training sits at the top of onboarding for workers. Facilities that fail audits risk shut-down; regular air sampling and surface residue checks keep everyone accountable.
Engineers reach for this silane daily in composites and coatings. The fiber-reinforced plastic panels in city buses or wind turbine blades hold up to weather because 3-Methacryloxypropyltrimethoxysilane builds a bridge between rough glass fibers and tough polymer resins. Bond lines in structural adhesives, marine caulks, and construction sealants grow more resistant to water, chemicals, and physical stress thanks to a reliable primer coat based on this molecule. You’ll spot its use in electronics—the circuit board world prizes it for creating moisture-resisting coatings that don’t delaminate. Dentists recognized the value in the 1990s, using it on glass ionomer and ceramic surfaces so fillings stick longer. Even low-VOC coatings and advanced paints adopt the silane for its weathering performance.
Lab benches and pilot plants keep finding new uses for this molecule. Researchers at academic centers tinker with MPTMS for developing biomaterials, using it to customize surfaces in tissue engineering and drug delivery. Studies on nanocomposites grow each year, with papers linking higher silane loading to stronger, lighter plastics for the automotive and aerospace industries. Material scientists report consistent progress in making safer, more reliable silane-based primers by customizing molecular structure and testing their behavior under extreme temperatures or wet cycles. Publications include data on changes in adhesion, water contact angle, and mechanical fatigue. Conference posters spill over with novel applications in sensor technology, solar cells, and environmental coatings, showing no sign of research interest fading.
Every chemical faces scrutiny once it leaves the plant. 3-Methacryloxypropyltrimethoxysilane’s toxicity profile draws from workplace exposure studies and animal tests. Most findings agree that it carries low acute toxicity in small doses, but repeated exposure to vapors or liquid can cause moderate skin and eye irritation. It isn’t classed as a carcinogen, but researchers highlight potential risks with chronic inhalation, especially where ventilation fails or spills aren’t cleaned quickly. Animal research has not shown major reproductive risks at typical workplace doses. Environmental fate remains under study: breakdown products, particularly methanol, need careful management in water streams. Regulators in the EU, US, and Asia each set unique tolerance limits and require complete reporting, especially as emerging research suggests nanoparticles bearing MPTMS coatings should be handled with increased caution until more data rolls in.
MPTMS stands at the crossroads of older composites and new "smart" materials. Demand grows as companies chase smaller, lighter, and tougher devices and structures, from 5G antennas to EV battery packs. Material suppliers look for ways to reduce VOCs and outgassing, challenging the industry to introduce renewably sourced silanes or develop variants that work under milder preparation conditions. R&D teams probe into using this silane for covalently binding bioactive molecules to implants, hoping to speed up healing and reduce infection rates. The molecule’s future seems laced with as much risk as promise—regulatory agencies worldwide keep one eye on occupational exposure and environmental impact as industries look to scale up. New automation in chemical manufacture pushes batch control and environmental compliance, shifting the focus from filling shelves to fitting into closed-loop green supply chains.
Walk into any lab or shop blending advanced coatings, adhesives, or composite materials, and the name 3-methacryloxypropyltrimethoxysilane often pops up. For something with such a mouthful of a name, it quietly shapes the modern world. I first saw its label on a drum stacked high in a resin facility, and it hit me that such chemicals don’t just collect dust—they drive progress.
Many industries run into the same problem: how to get things to stick together that normally keep their distance. Glass and plastic, metal and polymers—each have their own personalities, so just slapping them together means things often pull apart or degrade fast. Markets like automotive, aviation, and electronics want anything but failure in these bonds. That’s where this silane comes in. It brings together ingredients that usually don’t play nice.
On one side, the methacryloxy group loves teaming up with organic molecules in resins or plastics. On the flip side, those methoxy groups reach out to glass, ceramics, or mineral fillers. Once added to a formula, it hooks onto both surfaces, creating a chemical handshake that’s tough to break. The result? Stronger bonds that last longer in the real world, not just on paper.
I’ve seen first-hand that the silane’s role shows up in places people rarely notice. In construction, workers sometimes apply it to glass fibers woven into cement panels. Without it, those fibers might pull out, especially where you need reinforcement. In coatings, it helps paints or primers grab onto glass, aluminum, or silicon wafers. The electronics sector counts on that reliability because even the best gadget can go dark if layers peel apart. Boat builders, wind turbine makers, and car part engineers all keep an eye on durability, and this little silane keeps projects on track.
Stability and longevity get all the attention, but boosting resistance against heat, moisture, and scratching matters too. Silane-treated surfaces fear less from weather, saltwater, and heavy use. As a runner facing slick roads, I understand the sweat that comes with keeping things together; in manufacturing, the stakes sit high, and a surface that resists splitting or swelling makes life easier for everyone down the line.
Factories run smoother when ingredients mix more easily. Instead of clumping or floating, powders and liquids blend. Experienced folks in plastics and coatings tell me that silanes cut down on waste, so fewer batches fail. Costs add up if entire lines grind to a halt, and this helps keep the wheels turning.
Some manufacturers now look for cleaner, “greener” solutions, worried about long-term health and the planet. Silanes can help here too. By making adhesion more dependable, folks need less material and fewer reapplications, which in turn shrinks a company’s environmental footprint. That’s a win for business and the world outside the lab.
Nobody sees the billions of tiny bonds at work in a power line or medical sensor. Still, these connections shape our lives in countless ways. By pushing chemistries like 3-methacryloxypropyltrimethoxysilane forward, we get products made tougher, lighter, and more reliable. Companies investing in training and monitoring make sure everyone handles chemicals with care, not just for output but also for safety.
In the end, people expect the things they use every day—cars, phones, buildings—to last. Whether you spot a silane label in a backroom or not, its fingerprints stretch across industries, holding the future together.
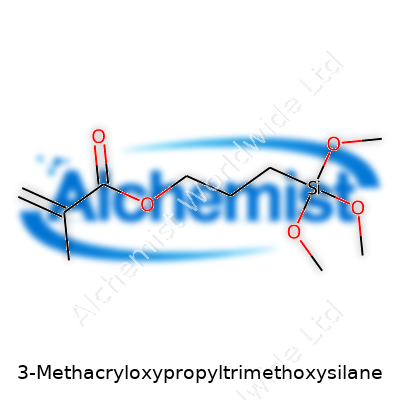
3-Methacryloxypropyltrimethoxysilane does not exactly roll off the tongue. In the world of chemistry and industry, though, names like this show up everywhere—from paint and coatings labs to dental material manufacturing. Digging into its chemical formula, you find something that packs a punch: C10H20O5Si. It tells a story about what this molecule brings to the table and why it pops up in both everyday products and advanced tech.
This silane compound hooks together two worlds in a single structure. Its methacrylate group—the familiar backbone of plastics and dental resins—allows for strong, durable connection with organic materials. The trimethoxysilane tail makes it a team player with minerals, glass, ceramics, and stone. All of this comes together in C10H20O5Si.
Thinking about my own use of materials in construction and repair jobs, I always looked for something that could keep stuff sticking under stress. This kind of compound delivers reliability, whether sticking resin to a countertop or bonding paint to a metal door frame exposed to sleet and sun.
It’s easy to lose sight of what’s important in a chemical formula. Methacryloxypropyltrimethoxysilane shows its worth not through fancy jargon, but through toughness and adaptability. Silane coupling agents like this help plastic and fiberglass blends stand up to moisture and dirt. Window sealants, for example, last longer in tough climates because of the way this molecule links glass to polymer.
Dental technicians rely on it too. Most people have had a tooth repaired eventually—strong bonds between composite resin and tooth happen because of small details in chemistry, not luck. That’s where this formula steps in and gets results, not just in a test tube, but in every bite and every smile.
Looking up the numbers helps cement trust. Studies show that silane coupling agents like 3-methacryloxypropyltrimethoxysilane reduce water absorption and increase shelf life in finished products. Industrial reports and patent filings back this up with thousands of commercial uses since the 1960s. The benefits find support in both lab data and day-to-day performance, measured not only in hours of wear, but in dollars saved on replacements and repairs.
Everything, including this silane, brings challenges. The formula includes three methoxy groups—those can allow unwanted chemical reactions if moisture control slips during storage or use. Manufacturers need to stay sharp and use airtight containers, train workers on safe handling, and monitor quality tightly.
Some communities have air quality concerns from using silane-based coatings. Switching over to greener solvents or water-based processing can help, but the solution to safer use starts on two fronts: better training and real investment from companies to upgrade outdated equipment.
As someone who prizes reliability, I know the best payoff comes from both understanding the formula and sticking to safety and careful handling. Collaboration between chemists, workers, and end users pushes these products towards safer, smarter use. Paying attention to both the power and pitfalls of formulas like C10H20O5Si gets everyone closer to materials that not only last, but make healthier workplaces and homes.
Over the years, I've seen plenty of people give little thought to where or how chemicals get stored. Some imagine a dark shelf in a back closet does the trick for everything. That approach invites disaster—especially with substances like 3-Methacryloxypropyltrimethoxysilane. This chemical brings a hefty dose of volatility. Left in the wrong conditions, it causes headaches ranging from ruined product to real danger.
Heat and moisture turn this silane into a time bomb. Once you open a drum or a bottle, air and humidity invite premature hydrolysis. Over time, the liquid becomes cloudy or forms a gel—if that's in your process line or stored goods, you're tossing out both product and money. No one enjoys explaining waste to the boss.
Keep this material tucked away between 5°C and 30°C. I always push for cool, dry spaces with steady temps. Some labs put even tighter controls on their climate—think refrigerators for smaller containers, or insulated, ventilated cages for drums. The extra effort upfront prevents costly headaches later.
Every time humidity sneaks in, you shave off some of the chemical’s shelf life. After opening, I reach for nitrogen or dry air to blanket the container before sealing it up again. Those little packets of desiccant packets in storage bins? They're there for a reason. Toss a couple in, and you'll thank yourself the next time you pour.
After I pop a cap, I close it right away. No conversations, no distractions—just tighten it up. Leaky lids never make for happy labs.
Direct sunlight speeds up decomposition. I've learned to store this stuff far from any windows or glass doors. Some folks use amber containers or stash their drums behind partitions to block UV rays.
Stainless steel and glass usually work for long-term storage, but I have seen cheap plastics leach or interact with the chemical—so watch your container choices. Some materials just don’t hold up, and nobody wants to clean up after an unexpected mess in the storage bay.
Sharing space means trusting coworkers to remember what sits on each shelf. I always label containers with open dates, lot numbers, and my own initials. If someone finds a leak or off smell, they know right away whether to toss the batch or give a heads-up.
Every worksite should line up spill kits, PPE, and clear signage wherever chemicals sit. I never skip training on storage and handling—assuming everyone just knows the rules courts disaster.
Attention to detail pays off in chemistry. By setting up a controlled, dry, and secure spot for 3-Methacryloxypropyltrimethoxysilane, you dodge unnecessary risk. For me, it's a small insurance policy for the team, the process, and the bottom line. Smart storage isn't just for the rulebook—it's real-world wisdom.
3-Methacryloxypropyltrimethoxysilane, often used as a coupling agent or adhesion promoter, shows up in a surprising number of products. Paint, sealants, rubber, and resin formulations rely on it to help surfaces stick together. Factories and labs appreciate its efficiency, but people don’t always pause to consider the impact on health.
I’ve handled chemicals like this in industrial settings. Even when the label looks full of warnings, folks sometimes shrug them off. That kind of thinking can put you in danger. For this silane, safety data sheets point to several clear risks. Direct contact with skin may cause irritation and redness. The vapor can sting your eyes or trigger headaches if you breathe enough in a poorly ventilated room. Splashes should never reach your eyes — the risk of severe irritation is very real.
This is not a chemical you want inside your lungs. Animal studies reveal that prolonged inhalation causes problems in airway tissues. Repeated exposure can lead to a chronic cough or a nasty bout of bronchitis. Some research suggests that organosilanes might act as sensitizers, leading to allergic reactions after repeat encounters.
As for toxicity, 3-methacryloxypropyltrimethoxysilane falls short of acute deadly poison, but even moderate chronic exposure has consequences. The European Chemicals Agency lists it as hazardous, and U.S. manufacturers must label it as an irritant, so these concerns aren’t just speculation.
During application or manufacturing, those most at risk handle silanes directly. Simple mistakes can make a big difference. Forgetting to wear gloves or goggles — easy to do during a hectic shift — leaves you open to burns and rashes. Factories must install good ventilation, especially in closed spaces, to avoid inhaling vapors. In my own work, the smell alone sets off alarm bells. I’ve watched seasoned workers get careless, only to regret it when their eyes started watering or their skin broke out.
Even hobbyists working with resins or paints at home can run into trouble. The temptation to skip protective gear is strong outside an official workplace. Unfortunately, that’s just when problems sneak up. Sensitization can start out as a mild itch and turn into a lifelong sensitivity.
Training makes all the difference. Health and safety routines only matter if people follow them. Gloves, goggles, and good ventilation aren’t optional, no matter the setting. Firms talking about “compliance” need to go beyond paperwork and focus on real protections on the shop floor.
Spills and splashes should get immediate cleanup, with proper waste disposal. People need to know how to use safety showers and eyewash stations. Labeling goes a long way, but so does keeping untrained hands away from storage cabinets. Communication beats assumptions every time.
Science keeps searching for safer alternatives, but progress takes time. Meanwhile, everyone from manufacturers to hobby users should pay serious attention to handling. Regular training and real enforcement keep health risks in check. The cost of a pair of gloves or a decent mask feels tiny compared to the price of long-term illness. Simple habits have saved more skin than any chemical innovation so far.
In the world of manufacturing and science, things rarely work together by accident. Take plastics and glass. Stick them side by side, and you'll notice they don't always form a strong partnership. This is where 3-Methacryloxypropyltrimethoxysilane proves its worth. This mouthful of a molecule acts like a translator for materials, connecting resins and fillers that usually don’t get along.
Walk through any airport and run your hands along a passenger jet. Beneath that smooth exterior, you'll find composite materials that rely on silane coupling agents. 3-Methacryloxypropyltrimethoxysilane coats glass fibers before they meet the resin matrix. The goal isn’t just sticking them together. It’s about squeezing out every possible bit of mechanical strength, so engineers can design lighter, tougher airplanes and cars. Without that molecular handshake, composites lose their edge—literally and figuratively. The same goes for fiberglass boats, wind turbine blades, and advanced tennis racquets.
For anyone who’s painted a bathroom, you know steam and moisture test every coat. 3-Methacryloxypropyltrimethoxysilane finds a home in paints and protective finishes that can’t afford to peel or flake. It locks pigments and polymers onto glass, concrete, and even metal surfaces. The result moves beyond surface-level bonds. With the treatment, the finish can shrug off weather, reduce cracking, and keep colors sharp. Out in the real world, this keeps bridges and skyscrapers looking new, cutting down on maintenance and repairs.
Open up a smartphone, and you’ll see a maze of circuit boards, contacts, and silicone gels. Reliability depends on unwanted moisture staying out. 3-Methacryloxypropyltrimethoxysilane gets used to treat surfaces inside these devices and in the silicone sealants that fill tiny gaps. You end up with better adhesion and lifelong water protection. Without such steps, electronic failures aren’t just possible—they’re nearly guaranteed, especially in harsh climates or high-humidity environments. Factories see real cost savings by keeping products alive longer instead of replacing them early.
As useful as these silanes are, they don’t always play nicely with every resin, nor do they stay stable on the shelf forever. Manufacturers pay close attention to storage conditions and mix ratios. Using the wrong amount or blending with the wrong chemicals leads to inconsistent results. Stories from labs show technicians double-checking levels and monitoring batch quality like hawks. Some companies turn to analytical tools, such as gas chromatography, to confirm the right silane makes it into each process. This stops bigger, more expensive failures further down the line.
Experience in material science labs shows that knowing what happens at the molecular level can prevent a lot of trouble. Training shop-floor workers to spot clumps, run simple tests, and work with raw materials safely pays dividends. Industry feedback keeps pushing suppliers toward formulas that work better, last longer, and pose fewer health risks. Better ventilation, smarter handling techniques, and focus on personal protective equipment help create a safer environment for workers exposed to chemical additives like 3-Methacryloxypropyltrimethoxysilane. Responsible use today will help preserve these advances for the future of construction, transportation, and technology.
| Names | |
| Preferred IUPAC name | 3-(Trimethoxysilyl)propyl 2-methylprop-2-enoate |
| Other names |
3-(Trimethoxysilyl)propyl methacrylate γ-Methacryloxypropyltrimethoxysilane Methacryloxypropyltrimethoxysilane MPS A-174 |
| Pronunciation | /ˌθriː mɛˌθæk.ri.ˌlɒk.siˌproʊ.pɪl traɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 1465064 |
| ChEBI | CHEBI:60137 |
| ChEMBL | CHEMBL1876751 |
| ChemSpider | 2057309 |
| DrugBank | DB14058 |
| ECHA InfoCard | 03e2e906-aefd-466e-9d36-6d33f8b73cee |
| EC Number | 203-474-9 |
| Gmelin Reference | **80816** |
| KEGG | C19688 |
| MeSH | D016195 |
| PubChem CID | 8752 |
| RTECS number | VV6175000 |
| UNII | T2VTW58N98 |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C10H20O5Si |
| Molar mass | 248.36 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Sharp |
| Density | 1.045 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.2 |
| Vapor pressure | 1 mmHg (169 °C) |
| Acidity (pKa) | 1.7 |
| Basicity (pKb) | 4.5 |
| Magnetic susceptibility (χ) | -7.58×10^-6 cm³/mol |
| Refractive index (nD) | 1.429 |
| Viscosity | 10 mPa·s |
| Dipole moment | 3.05 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -657.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | '-7347 kJ/mol' |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Precautionary statements | Precautionary statements: P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 79 °C |
| Autoignition temperature | 290 °C (554 °F; 563 K) |
| Lethal dose or concentration | LD50 (Oral, Rat): 7,346 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 8,700 mg/kg |
| NIOSH | NIOSH SN9846000 |
| REL (Recommended) | 0.1-0.2 |
| Related compounds | |
| Related compounds |
3-Glycidoxypropyltrimethoxysilane Vinyltrimethoxysilane Methyltrimethoxysilane 3-Aminopropyltriethoxysilane Methacryloxypropyltriethoxysilane |