
Chemists always look for bridge-builders, and 3-Methacryloyloxypropyltrimethoxysilane (often known by the shorthand KH-570, A-174, or by its CAS Number 2530-85-0) proves just how inventive the materials science world can get. Decades ago, when silane coupling agents started gaining ground, the focus was simply on improving adhesion between organic resins and inorganic fillers. As the plastics and composites industries grew, the field demanded something that could mesh acrylic chemistry with the tough, unyielding world of glass fibers and minerals. In the mid-20th century, researchers combined methacrylate and trialkoxysilane chemistries, producing this hybrid molecule that soon became a staple in adhesives, coatings, and glass fiber sizing. Over time, as cross-disciplinary teams in Europe, the US, and Asia shared results, data poured in showing just how well 3-Methacryloyloxypropyltrimethoxysilane could boost performance in moisture-prone, temperature-stressed, and vibration-heavy jobs.
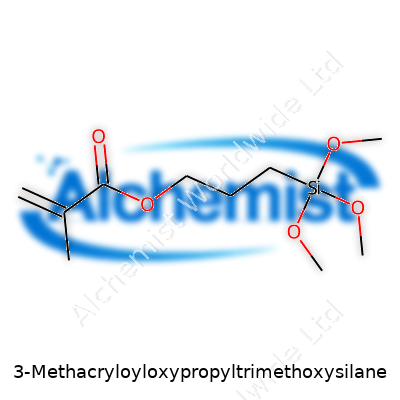
At its core, 3-Methacryloyloxypropyltrimethoxysilane ties together an organic methacrylate group with a trimethoxysilane group, opening the door for it to bond across very different surfaces. Producers supply it as a clear, slightly yellowish liquid, usually with a sharp alcohol-like odor. Its main job sits in improving the bond between polymers and inorganic surfaces. Take industries like construction, electronics, or automaking; many rely on composites containing both resin and filler. Without a bridge molecule like this, layers can peel, or cracks can creep in where resin meets glass or metal. Research teams worldwide have demonstrated—over hundreds of patents and published papers—why mixing this silane into epoxy or polyester resins pushes the limits for things like water resistance and fracture toughness.
3-Methacryloyloxypropyltrimethoxysilane carries the chemical formula C10H20O5Si, with a molar mass just a shade below 248 g/mol. The boiling point lands near 295°C, but long before that, you’ll notice it hydrolyzes in water, especially in acidic or basic environments. What really stands out is its dual-reactivity: the methacrylate end takes part in free-radical polymerization, while the trimethoxysilane end hydrolyzes to form silanols. These silanols create stable bonds with glass, metal oxides, and other inorganic surfaces. Handling the liquid—its viscosity sits around 2-3 mPa·s at 25°C—means working with gloves and goggles, as it vaporizes enough to bring eye and respiratory irritation risks.
Commercially-supplied material usually guarantees a purity above 98%. Companies like Dow and Evonik provide it in drums or intermediate bulk containers, always sporting hazard labels to mark its flammability and health irritant status. SDS sheets list it under GHS classification for skin and eye irritancy. On product datasheets, look for information such as density (about 1.04 g/cm³ at 25°C), boiling point, flash point (around 108°C), refractive index (about 1.430), and water solubility (reacts, generating methanol). Shipping companies keep strict paperwork on it due to its UN 1993 flammable liquid classification.
Manufacturers typically synthesize this silane by reacting methacrylic acid or its methyl ester with 3-chloropropyltrimethoxysilane or by transesterifying 3-hydroxypropyltrimethoxysilane with methacryloyl chloride. Catalysts, solvents, and temperature controls play a huge role in cranking up yields and limiting byproducts. After reaction, most producers distill the crude product under reduced pressure to hit the high purity demanded by the electronics and composite sectors. Residual acid or chloride can corrode metal surfaces or trigger unwanted cross-linking, so tight analytic controls keep these at low ppm levels.
The molecule’s true power comes in how many ways the two functional ends can react. On one side, the methacrylate group can join acrylic, epoxy, or unsaturated polyester networks through free-radical or ionic mechanisms. On the other, under the right water and pH conditions, the trimethoxysilane groups hydrolyze, condense, and attach covalently to metal oxides or glass surfaces. Scientists have explored modifying its structure—switching methacrylate for other vinylic groups, or altering the silane’s alkoxy side—for steeper or gentler reactivity curves. Bulk applications often blend it with other silanes, titania, or alumina nanoparticles, creating covalently-linked hybrid networks inside coatings or casting resins, making the bridge even stronger.
Industry folk may refer to this substance with a slew of names: KH-570, A-174, Z-6030, Sila-Ace S210, or by its IUPAC moniker, 3-(Trimethoxysilyl)propyl methacrylate. The label matters for procurement—as supply chains run across China, Germany, and the US, buyers need to recognize all these synonyms to snag the real stuff. Major catalogs organize it under silane coupling agents, special adhesives, or even under the broader category of “surface modifiers.” If you see a bottle labeled “Silquest A-174” or “Momentive Z-6030,” you’re looking at this molecule through a branding lens.
Most labs and manufacturers working with this silane enforce solid engineering controls: fume hoods, eye-wash stations, and PPE for anyone handling open drums. Flashpoint sits low enough that sparks or static discharge create real risks in bulk storage. Any spill needs immediate cleanup with absorbents, avoiding ignition sources. Most factories run leak checks and ventilation audits, since vapor inhalation or repeated skin contact can cause dermatitis or chemical burns. Waste streams containing hydrolysis byproducts go to controlled incineration or chemical neutralization setups. Over the years, studies from agencies like NIOSH and ECHA have outlined clear guidelines—remind workers to wash thoroughly and change contaminated clothing right away.
Across industries, users rely on 3-Methacryloyloxypropyltrimethoxysilane for one chief reason: better adhesion. In pultrusion shops making fiberglass window frames, just a drizzle boosts the stickiness between polyester resin and the glass. In adhesives, it reduces water uptake at the bond line, cutting down on swelling or debonding in wet conditions. Coating formulators find that adding 1-2% by weight locks in anti-corrosion properties and keeps films smooth over concrete, aluminum, or steel. Even in dental composites, where filler isn’t sticking to resin, you’ll likely find its methacrylate-silane combo hidden in the MSDS. Electronics designers pick it to prep glass or ceramic surfaces before mounting chips or printing circuits, avoiding the delamination problems that tank yields.
Technologists in composites, electronics, and specialty polymers keep pushing this molecule in new directions. Over the last decade, projects have measured how blending this silane with nanoparticles increases barrier strength for food packaging or power cable insulation. New patents describe tri-silanes that shoot for more hydrophobic or oleophobic endings, shifting the durability of marine or automotive parts. Academic teams track how it interacts with carbon nanofibers, seeing jumps in mechanical performance when added to epoxy matrices for aerospace brackets. Uniform characterization of its hydrolysis kinetics, shelf-life under different climates, and reactivity in composite blends keeps chemists chasing the best dose, pH, and blending order for every job.
Concern over exposure to silanes has led to thorough studies in animal and cellular models; several results show low acute toxicity via oral or dermal routes, but vapor inhalation demands solid controls. Eye and skin irritation happen at low concentrations, prompting calls for upgraded PPE among factory workers. Methanol produced during hydrolysis is a key hazard, known for both acute toxicity and chronic risks. ILLOSA and ECHA guidelines limit workplace exposure using air monitoring and biological sampling. Workers and regulators now track exposures to byproducts, especially where downstream incineration or water treatment might let residuals escape into the environment. Researchers still monitor chronic and environmental impacts due to potential persistence and breakdown into silica nanoparticles or organic fragments.
With each year, environmental and durability expectations rise. The push toward halogen-free, low-voc, and circular economy materials challenges formulators to lean on stable, high-performance coupling agents like 3-Methacryloyloxypropyltrimethoxysilane. Plant-based and bio-polymer composites demand new silane blends that deliver the same toughness without toxic solvents or high waste. R&D teams hunt for versions that work on trickier fillers—like metal-organic frameworks, recycled glass, or natural fibers—making the molecule work in more “green” applications. Automation in fiber sizing, resin prep, and adhesive blending will likely change the workflow, but technical staff will still count on clear, reliable silane chemistry. As global supply chains stretch, authentication and traceability of raw materials grow in importance—every bottle and drum needs rock-solid documentation to avoid counterfeiting. Where manufacturers and research labs keep sharing results, 3-Methacryloyloxypropyltrimethoxysilane stays relevant, right at the dynamic edge of modern engineering and materials design.
Plenty of folks in manufacturing and research cross paths with 3-Methacryloyloxypropyltrimethoxysilane, even if the name sounds like something that belongs in a chemistry Olympiad. Anyone who’s dealt with composite materials, improved coatings, or modern adhesives has probably benefited indirectly from it. If you’ve ever driven a car with smooth finishes or used dental fillings that actually last, this stuff likely played a part.
At its core, this chemical acts like a matchmaker between glass, metals, or ceramics and all sorts of synthetic resins. Surfaces don’t always mix well with plastic-based materials or coatings. Drop this silane into the equation, and they suddenly cling together a lot better. Think about auto windshields that hold on tight to their sealants, or glass-fiber reinforced plastics that don’t crack under pressure. Silane couple agents, such as this one, change how industrial materials stick together – and that reliability means fewer product failures and lower costs for everyone down the line.
I’ve seen small businesses grow when they started building parts with composite materials treated with this silane. In the lab, we used it as a primer to help toughen up laminated plastics before sending them through the furnace for stress testing. The science behind it isn’t just for show: studies reveal that 3-Methacryloyloxypropyltrimethoxysilane improves mechanical strength, boosts moisture resistance, and extends product life. Automotive panels, construction sealants, electronic circuit boards — lots of big-ticket industries trust this tiny molecule.
Like any chemical, it brings both benefits and responsibilities. Exposure can irritate skin or eyes, so safe handling matters. Facilities storing this chemical train their staff, keep inventories tight, and run ventilation systems by the book. The world’s stricter regulations for chemical safety, such as REACH in Europe and TSCA in the U.S., cover how companies buy, store, and dispose of silane compounds.
Some folks worry about how silane-treated materials interact with recycling streams. Disposing of fiber-reinforced composites works best when all the ingredients can break down or get repurposed without trouble. Manufacturers experiment with water-based silane formulations to limit volatile emissions during production. Environmental studies show that switching out solvents with safer alternatives keeps air quality better around production plants and trims hazardous waste.
Tomorrow’s composites and coatings push for lower environmental costs while still delivering strength and durability. Material scientists try tweaking the molecular structure of silanes to work at lower concentrations, unlocking cost and sustainability wins at once. For consumers, these innovations mean products that break less, weigh less, and leave a smaller footprint.
So, 3-Methacryloyloxypropyltrimethoxysilane doesn’t draw crowds, but it strengthens the backbone of several big industries. Solid knowledge about its uses and risks puts safer, longer-lasting, and sometimes greener products on shelves and roadways everywhere.
Anyone who’s handled industrial chemicals knows that the storage question isn’t just a box on a safety checklist. A compound like 3-Methacryloyloxypropyltrimethoxysilane—used in everything from adhesives to advanced composites—brings unique risks for both workers and products if the basics of storage get ignored. Underestimating safe storage causes headaches that stretch from ruined materials to serious health hazards. I’ve seen it: overlooked leaks, unnecessary exposures, and damaged inventory because someone left a canister in the wrong corner.
This material doesn’t play nice with water. Its silane backbone reacts pretty aggressively when exposed to moisture, which means humidity or even small spills can set off unwanted chemical changes. The surface of a bench or a forgotten open lid will speed up hydrolysis and leave you with degraded, useless product. Containers should live in a moisture-controlled spot, tightly sealed. Racking drums or bottles above floor level helps streamline leaks and cleanup, but also limits exposure to rising humidity.
Sunlight is another culprit. Direct hit from sunlight starts breaking down this chemical fast—ultraviolet rays scramble its molecular structure, which means the active parts you paid for disappear before you ever open the drum. Opaque or amber containers take out half the battle, and solid doors on storage rooms do the rest. Every time I’ve seen someone store this in a sunlit warehouse, the remediation costs have reached into the thousands.
Temperature swings matter more than most people guess. Below 15°C (59°F), the compound thickens, which makes pumping annoying and can even turn part of a batch solid, forcing disposal. On the high end, passing 25°C (77°F) speeds up contamination and increases pressure in sealed packaging—a risk for sudden spills. So it’s worth investing in a stable, indoor space with air conditioning or at least dependable insulation.
Rules mean little if folks in charge of storerooms and shipments shrug them off. Every time a shipment goes out of spec, production lines stall and the finger-pointing starts. The best teams hold real discussions about what storage looks like in their space, using hands-on training that highlights accident stories—and trust me, I’ve sat through a few of those gut-wrenching postmortems.
Safety data sheets point out incompatibilities, so cross-contamination shouldn’t surprise anyone. This silane reacts with acids, bases, and oxidizers. Even a trace amount of a cleaning agent or an improperly rinsed drum starts trouble. I’ve always pushed for labeling zones and physical barriers in mixed storage, not just signs.
Investing in airtight, chemical-resistant containers cuts headaches by half. Inventory checks, tracking every drum’s location and condition, can catch a bad batch before it reaches someone’s workstation. Double-checking date-of-manufacture avoids expired surpluses stacked in the back corner, forgotten until it’s hazardous waste.
Regular shelf-cleaning and surface checks give an early warning on leaks. Sensors to track ambient moisture keep surprises low, but nothing replaces a sharp eye and the willingness to pause production to fix small issues before they escalate.
Good storage doesn’t just check off legal boxes—it’s about creating a safe, productive shop floor where costly mistakes don’t eat through bottom lines or put people at risk. Smart habits, simple tools, and real communication lower the odds of learning about 3-Methacryloyloxypropyltrimethoxysilane the hard way.
Working with 3-Methacryloyloxypropyltrimethoxysilane brings a unique set of hazards that call for more than just a passing glance at the safety data sheet. This chemical, common in coatings and adhesives, gives off vapors that can irritate the nose, throat, and lungs. It can sneak past basic gloves and sting your skin, or leave your eyes burning after a careless splash. So many workers have learned the hard way that skipping protection in favor of speed means more than just a red mark by the clock.
Gloves do the heavy lifting here, but not all gloves work the same. Years in a lab taught me that nitrile holds up much better than latex when dealing with organosilanes. A colleague once tried latex and ended up with a rash and wasted product—cheap gloves cost more than good ones. Safety glasses or goggles with side shields stop accidental sprays. For breathing, a well-fitted mask with organic vapor cartridges keeps harmful fumes out when work goes beyond popping a cap open. Clothes should cover arms and legs, preferably a lab coat that gets washed regularly, not left hanging near a workbench.
It’s tempting to crack a window and hope fresh air will push fumes away, but true protection comes from proper extraction. I spent too many hours in stuffy rooms with only box fans before learning that a chemical fume hood makes a huge difference. Silane vapors like these settle in the lungs and linger on surfaces. Local exhaust means less chemical floating around and less after-work headache. Labs with good airflow and spot ventilation give peace of mind and let you focus on the task instead of worrying about what you’re breathing in.
This liquid reacts swiftly with humidity and can release methanol—a toxic substance in its own right. Keeping containers tightly closed, in a dry and cool spot, makes sense. Friends in the field have plenty of stories about sticky messes and mystery smells from barely closed bottles jammed next to acids or bases. Only store what you plan to use in the short term and make sure labels stay readable, not faded or peeling.
Even seasoned staff slip up. I remember one morning that started with a minor spill but could have spiraled out of control without proper absorbents and neutralizers at hand. No one plans to drop a bottle, but being the person who calmly grabs spill kits instead of panicking says a lot about real preparedness. Dispose of any contaminated cleaning materials as hazardous waste: not doing so can put others at risk, down the line.
It’s all too easy to overlook safety when days get busy. Enthusiasm and careful training beat cutting corners. Newcomers should not hoist bottles solo until they have shown they understand the rules. Short videos, checklists taped to benches, and open conversation about mistakes help keep safety fresh. Commitment starts at the top, from supervisors who set a hard line on proper handling and regular safety briefings.
Looking back, it’s clear that building safe habits matters every bit as much as choosing the right glove or hood. Nobody wants to end their shift with a rash, a cough, or worse. Local exhaust, proper gear, well-marked storage, and active training—these protect both the person and the lab. Every worker deserves to finish the day as healthy as they started.
At the core of many materials — from composites to advanced coatings — you’ll find a blend of organic resins and inorganic fillers. Companies and researchers have tinkered with the gap between these two worlds for decades. The go-to solution remains silane coupling agents, which help the different ingredients touch base at a molecular level. One compound that keeps popping up in lab reports and patents is 3-Methacryloyloxypropyltrimethoxysilane, better known as MEMO.
MEMO features a methacrylate group on one end and trimethoxysilane on the other. This structure lets it grab onto both glass or metal surfaces and the backbone of unsaturated polyester or acrylic resins. I’ve seen MEMO pull off smoother interfaces when you’re working with fiberglass-reinforced plastics. There’s a boost in mechanical strength and a more durable finished product.
In the real world, few production lines stick with one silane. Formulators often add other silanes to the kettle to balance price, shelf-life, or application quirks. Methyltrimethoxysilane or γ-glycidoxypropyltrimethoxysilane sometimes join in. This is where things get tricky. Not every silane plays nice in the pot with MEMO. Chemical structure differences create rivalries. Poor handling results in hydrolysis or premature condensation, leading to inconsistent product quality.
I learned to check not only the chemical compatibility on paper but also how mixtures behave before scaling up. Hydrolysis rates differ and cross-reactions can reduce the available active groups. Strong alkoxysilanes or amino-silanes may cause faster gelation or affect long-term storage. The key lesson: thoroughly test formulations under realistic storage and processing conditions.
Chemical compatibility with resins ranks just as high on my checklist. MEMO forms bonds with resins that have unsaturated double bonds, like acrylics or polyesters. When you throw it into epoxies or polyurethanes without careful design, bonding doesn't always reach the same level. Water-based systems throw up more roadblocks. Side reactions and issues with hydrolytic stability surface if you skip compatibility testing.
To reduce problems, research points to using low concentrations of MEMO and thorough mixing. A little patience goes a long way. Sometimes a small adjustment in pH or solvent lineup makes the difference between a streaky dud and a strong, stable composite.
Reliable data supports experimentation. In recent years, reports from the Journal of Applied Polymer Science and a handful of technical data sheets agree that MEMO interacts well in specific blends, but results vary by co-silane type and resin chemistry. MEMO used alongside aminosilanes can produce excellent adhesion in glass fiber composites—if the process sequence and pH get extra attention. In contrast, trying to blend multiple methacrylate silanes can spark early gelation or reduce activity.
For anyone troubleshooting a tough blend, the answer usually comes from simple, targeted tests under cold and hot conditions. I always stress documentation: measure viscosity at intervals, test shelf stability, and monitor changes in surface energy on treated fillers. Companies with strong technical support help by offering pairing advice based on published research.
Investing in better mixing and dosing equipment pays off. By keeping silanes dry until the moment of mixing, and using modern process controls, production lines get more consistent results. These real-world steps beat impressive-sounding formulae that don’t translate once the tanks ramp up to industrial volumes.
My experience, and the pattern in literature, shows that MEMO holds plenty of promise but demands respect for chemistry, careful testing, and process discipline. While formulators chase better, greener products, balancing silane and resin compatibility will remain a running conversation—one that keeps labs and factories experimenting, learning, and adapting month after month.
3-Methacryloyloxypropyltrimethoxysilane, known to many as a silane coupling agent, sometimes shows up in places I least expect. I’ve mixed paints and played with epoxies on weekend projects, not realizing a silane like this can help glue the base materials together behind the scenes. If you walk into a hardware store and grab a high-end adhesive or a premium glass coating, there’s a fair chance this compound or its cousins are on the ingredient list.
This chemical rolls out as a colorless to pale yellow liquid. I’ve noticed that it doesn’t smell strong, but like most organosilanes, it ought to be handled with decent ventilation. Its density is around 1.04 grams per cubic centimeter, so by weight it sits between water and standard cooking oils. It flows reasonably easily, and doesn’t feel sticky. With a boiling point of about 310°C, it isn’t going anywhere in a hurry during most processing. It’s also not quick to catch fire, having a flash point in the neighborhood of 110°C. Brushes and surfaces that touch it can be cleaned if you get to them before it cross-links or reacts with water in the air.
Most folks don’t get into the chemistry unless they’re in a lab, but this one has some neat tricks. On one end is the methacrylate group, eager to join in polymerization—like acrylics or vinyl made in plastic manufacture. On the other side, you find three methoxy groups attached to silicon. Give it a little water, and these methoxy groups will swap out their -OCH3 for -OH groups, turning the molecule “sticky” in a different way, letting it bond with glass, stone, metal, or ceramics. This double life of surface and polymer compatibility is why it’s in so many modern materials.
I’ve seen how this chemical settles disputes at the boundary between organic polymers and inorganic materials. Without it, paints flake off, adhesives don’t last, and coatings fail sooner. It forms a molecular bridge, not just sticking but actually bonding sides together so they hold up under strain, heat, or moisture. Its hydrolytic reactivity also warns me to keep it dry and sealed; even small leaks in a container pull in humidity from the air, which can spoil an entire batch before it’s used.
In practical terms, industries rely on this kind of molecule for long-lasting composites and better-performing products. On a basic level, hospitals benefit because their glass instruments and tools rely on coatings made stable by these silanes. In construction, reinforced glass and specialty adhesives owe a fair bit of their toughness to this bridging ability. Even personal electronics—those with fancy glass covers or fiber optics—wouldn’t keep pace without such stable surface modification.
We need mindful handling to protect workers’ skin and eyes. Knowledge helps: gloves, goggles, and proper containers make all the difference. The chemistry pushes for better efficiency in mixtures, so less waste and stronger bonds mean less maintenance down the line. I keep thinking about how improvements in formulations could save resources or reduce failure rates, not just in big factories but in smaller, hands-on shops too. Finding safer variants or even recycling approaches for silane-tainted products could offer an extra layer of sustainability, a goal plenty of us can get behind.
| Names | |
| Preferred IUPAC name | 3-(Trimethoxysilyl)propyl 2-methylprop-2-enoate |
| Other names |
KH-570 A-174 Dynasylan MEMO MEMO Silane coupling agent KH-570 3-(Trimethoxysilyl)propyl methacrylate |
| Pronunciation | /ˌθriː.mɛθ.əˌkraɪ.lɔɪˌlɒk.siˌproʊ.pɪl.traɪˌmɛθ.ɒk.siˈsaɪ.leɪn/ |
| Identifiers | |
| CAS Number | 2530-85-0 |
| Beilstein Reference | 4740617 |
| ChEBI | CHEBI:87137 |
| ChEMBL | CHEMBL3184852 |
| ChemSpider | 15392 |
| DrugBank | DB14060 |
| ECHA InfoCard | 03b88b77-089c-4afe-8ed2-6476ac1a3be2 |
| EC Number | 203-841-9 |
| Gmelin Reference | 87196 |
| KEGG | C14268 |
| MeSH | D017369 |
| PubChem CID | 87121 |
| RTECS number | OZ7276000 |
| UNII | K698M2DJ14 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID5020726 |
| Properties | |
| Chemical formula | C10H20O5Si |
| Molar mass | 248.34 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Characteristic |
| Density | 1.045 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | 0.3 |
| Vapor pressure | 1 mmHg (25 °C) |
| Acidity (pKa) | pKa ≈ 8.8 |
| Basicity (pKb) | pKb: 6.5 |
| Magnetic susceptibility (χ) | -6.25×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.429 |
| Viscosity | 6 mPa·s (25°C) |
| Dipole moment | 3.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 643.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -925.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7968.7 kJ/mol |
| Pharmacology | |
| ATC code | V04CX |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H318 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 79 °C |
| Lethal dose or concentration | LD50 (Oral, rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 8,710 mg/kg |
| NIOSH | GVG35000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methacryloyloxypropyltrimethoxysilane: "Not established |
| REL (Recommended) | 0.5-2.0 |
| Related compounds | |
| Related compounds |
Methacryloxypropyltriethoxysilane Vinyltrimethoxysilane 3-Aminopropyltrimethoxysilane Triethoxysilylpropyl methacrylate Trimethoxysilylpropyl methacrylate |