
Years back, chemists working with organosilanes kept bumping into the limitations of common coupling agents. Surfaces had trouble sticking together, whether they dealt with coatings, adhesives, or composites. Fast forward to the discovery and refinement of 3-ureidopropyltrimethoxysilane, a molecule that shook things up by bringing together the reactivity of silane with the versatility of the urea group. Studies from the late 20th century laid the groundwork, but real-world demand during the push for higher-performance polymers and specialty coatings turned this compound from a curiosity to a staple in labs and factories worldwide.
3-Ureidopropyltrimethoxysilane walks a line between different worlds. On one side, its silane backbone grabs hold of glass, metal, and mineral surfaces, fostering serious interfacial strength. On the other, the ureido group leans toward organic substrates and polymers. Researchers and industry veterans lean on this compound during the production of hybrid materials, high-durability paints, water-repellent treatments, and specialty adhesives for construction and automotive uses. Suppliers offer it in liquid form, usually with a faint smell and a clear to slightly yellow appearance.
Bringing this molecule into the lab, you get a clear, medium-viscosity liquid, boasting a molecular weight in the low 200s (often around 222 g/mol). The boiling point hovers near 270°C, though it decomposes before distillation can finish. The compound’s density settles around 1.13 g/cm³ at room temperature. Its trimethoxysilane group hydrolyzes easily under moisture, forming silanols and then crosslinked polysiloxane networks. The ureido end, with its propensity for hydrogen bonding, plays well with polyurethanes, epoxies, and other polar polymers.
Packaging and shipping stick to strict traceability and storage demands. Product drums clearly record batch numbers, purity (usually above 97%), water content (preferably below 0.1%), and the CAS number 23843-64-3. Labels highlight the need for storage away from humidity to avoid premature hydrolysis. Technical data sheets detail viscosity (ranging from 1.2 to 1.7 mPa·s), refractive index, and active content, because every batch comes with its quirks given raw material variability.
Factory chemists synthesize the compound by reacting 3-aminopropyltrimethoxysilane with potassium cyanate in a water-ethanol mixture, keeping the solution at moderate temperatures. This route turns the amine group into a urea derivative with well-controlled yield, usually over 90%. Purification involves extraction and vacuum distillation. It’s a straightforward process, but the details—like reaction time, pH, and the trickiness of safely handling cyanate—matter if you want solid yields and minimal byproducts.
After landing in the hands of chemists, 3-ureidopropyltrimethoxysilane sticks to a few main scripts. The trimethoxysilane end hydrolyzes and condenses on glass, ceramics, silica, and metal oxides. Its ureido side forms hydrogen bonds, linking up with hardeners or reacting with isocyanates and epoxy groups. Formulators sometimes tweak the ureido group or anchor the silane onto larger oligomers, tuning how it behaves in tough industrial applications. Surface modification shops use it to graft onto nanoparticles, boosting compatibility and durability in composites.
Old catalogs and regulatory lists sometimes call this compound N-[3-(Trimethoxysilyl)propyl]urea. Trade names crop up as A-1160, Silquest U-15, and others—differences that often come down to the supplier. Mixing up names can lead to frustrating errors, especially across international orders or when pulling up toxicology profiles from various regulatory sources.
Experienced users know that 3-ureidopropyltrimethoxysilane doesn’t go easy on skin and eyes. It can irritate mucous membranes, so gloves, goggles, and decent ventilation come standard when working with the liquid. Spills get cleaned up fast to cut down on slips and exposure, while storage rules keep drums tightly closed in cool, dry places. Waste disposal lines up with regulations for organosilanes, focusing on hydrolyzed byproducts. Many factories include it on their chemical safety training, stressing rapid washing after contact and careful handling of empty containers to avoid trace skin exposure.
Materials developers depend on this silane for its ability to boost adhesion in complex composite parts, whether those end up in wind turbine blades, high-performance circuit boards, or lightweight automotive panels. Builders see it in advanced sealants and long-life coatings that survive harsh outdoor conditions. The electronics world finds a use during the making of printed circuit boards where controlled moisture resistance and dielectric performance matter. Researchers keep finding novel uses for the compound in catalyst supports, hydrophobic surface treatments, and new generation filtration membranes that stand up to repeated chemical attack.
Academic and industrial R&D teams spend long hours tweaking how this silane gets used. University labs are pushing into nanocomposite realms, where the challenge lies in dispersing inorganic nanoparticles evenly in polymers—this silane enables just the right surface compatibility to get strong, flexible, and durable parts. Industry chemists mix it with other functional silanes to create multi-role coupling agents, delivering performance in tough conditions like marine paints or flexible solar panels. Real breakthroughs keep coming from sharing between disciplines, where surface science experts and polymer chemists swap ideas on silanization protocols.
Safety studies date back decades. Animal skin and eye tests show moderate irritation at higher concentrations, but no lasting injuries when rinsed away quickly. Inhalation exposures cause throat and lung irritation, yet no evidence currently points to long-term damage at low levels. Chronic toxicity looks low compared to other alkoxysilanes, but most users respect its potential for acute symptoms—wearing PPE and using well-designed ventilation. Disposal studies highlight that environmental breakdown products don’t build up, owing to rapid hydrolysis and conversion under wastewater treatment conditions. Always, regulatory bodies call for careful risk management and solid safety data sheets.
Sustainable materials keep climbing the priority list. 3-Ureidopropyltrimethoxysilane already pulls its weight by making stronger, lighter composites and durable, moisture-resistant sealants—these qualities lower replacement rates and cut waste. Looking ahead, the shift toward bio-composites, green adhesives, and circular economy products only grows stronger. Researchers see room for new derivatives with lower toxicity, higher reactivity, and tailored compatibility. Some are fine-tuning formulations to slash solvent content or drop processing temperatures, saving energy and improving operator safety. Industry players investing in next-gen surface modification, medical devices, and energy storage rely on the foundational chemistry of this molecule to keep standards high. Anyone betting on the future of advanced materials ought to keep a close eye on what happens in this corner of the silane market.
Let’s talk about the chemical 3-Ureidopropyltrimethoxysilane. It’s not a household name, but its footprint shows up in lots of places. In my years following the chemical industry, I’ve noticed how these specialty chemicals can change whole industries quietly, tucked away in the ingredients list. They might sound intimidating, but chemicals like these help build stronger bridges, safer cars, and even better homes.
This silane belongs to a group used as coupling agents. That means it plays a middleman’s game—helping two things stick together that ordinarily wouldn’t. Imagine trying to bond glass to plastic, or coating a metal surface so that glue or paint actually stays put and doesn’t peel off after a few months. That’s where the magic happens.
One area where 3-Ureidopropyltrimethoxysilane stands out is in the construction industry. Think concrete—builders worry about cracks, weather, and water damage. Adding this silane to concrete mixtures lets water-based coatings stick better to surfaces. Less peeling and flaking means longer-lasting buildings. You see similar use in sealants and adhesives—jobs that demand staying power, no matter the humidity in the air or spills on the floor.
This compound also crops up in the world of plastics and composite materials. Factories want to mix glass fibers with resins for car parts or sports equipment, but those materials don’t exactly get along. Using 3-Ureidopropyltrimethoxysilane helps the resin “grab” onto the fibers. This makes car bumpers or wind turbine blades more reliable for years.
I remember walking past some graffiti-resistant coatings in a trade show. What surprised me was that the rep pointed to a chemical like 3-Ureidopropyltrimethoxysilane as key to making the protective layer last through rain, baking sun, and the next clean-up. Chemically, this compound links with both the painted surface and the protective layer. That’s why these coatings shrug off abuse.
The electronics sector also benefits. Printed circuit boards have tiny tracks that must stay put and not corrode. Treating the boards with silanes like this helps insulate and protect them. Makers designing for rugged phones or outdoor devices pay attention here, chasing products that don’t quit after the first big downpour or temperature swing.
Safety can’t take a back seat. Handling silanes demands care. This one tends to release methanol as it reacts—a known irritant, and not a friend to respiratory systems. Workers rely on training, PPE, and good ventilation, following established safety protocols. I saw a production site years ago that invested in capture systems so nothing drifted into the walkways. That experience drove home how every big chemical advance gets paired with improvements in workplace safety.
People expect longer-lasting products. They want bridges and buildings to resist weather and wear. They look for paints and adhesives that hold on so families spend less time fixing and more time living. All of this draws on the unique properties of chemicals like 3-Ureidopropyltrimethoxysilane. Responsible manufacturers share data about their sourcing, environmental impact, and safety records. Sustainability reports and third-party audits keep companies honest.
I’d like to see the industry continue moving toward greener silane processes—reducing byproducts and improving the recycling of used materials. As advocacy for safer materials grows, regulators and companies can pull together, making sure tomorrow’s chemistry works for everyone.
Not every chemical on the shelf treats you the same way, and 3-Ureidopropyltrimethoxysilane definitely asks for some respect. If you’ve worked in a lab, you’ve probably seen folks rush to stash reagents anywhere that’s open, but this one has quirks that’ll make you think twice. The big problem is how it reacts if you just leave it out like salt or baking soda—moisture in the air creeps in, triggering a mess of hydrolysis that can gum up your experiment or foul an entire batch.
I once saw a rushed intern leave a bottle open in a humid storeroom. Next day, the silane inside had turned cloudy, and it gave off a sharp odor. That wasn’t a minor mishap. Silanes break down in water, and once that starts, you can forget about consistent results from that batch. The science supports this—materials safety data sheets advise dry conditions below 30°C, and remind users that these compounds often degrade quickly in ordinary air.
This stuff also emits methanol if it reacts, which isn’t just flammable, but toxic in the long run. So storing it far from heat sources, sunlight, or open flames isn’t just about extending shelf life. It’s about avoiding accidents that could hurt someone.
Over the years, coworkers have fallen into the “sealed container” trap: they picked any container and figured it was safe as long as the lid clicked shut. But not all containers block out moisture or air. Glass with a tight-fitting PTFE-lined cap or original packaging generally gives more reliable protection.
Anyone who’s kept supplies in a metal shed or by a south-facing window knows temperature swings quicken the breakdown of sensitive chemicals. I learned to keep all my silanes in a dry cabinet that logs temperature, away from direct heat pipes or vents. If the lab has a desiccator or purpose-built chemical refrigerator, that’s even better. Constant temperature means fewer surprises.
Good storage isn’t just about chemistry. It’s about planning, labeling, and teaching. In one lab, we started labeling “must keep dry” on silyl reagents, and logged dates of first opening. Folks checked records before reaching for a bottle, and mistakes dropped. Making sure containers go back promptly instead of sitting out all day helps too. Training new staff with clear rules—use gloves, and avoid switching bottle caps—builds habits that stick.
Chemicals like 3-Ureidopropyltrimethoxysilane reward good habits, not shortcuts. Long shelf life, reliable experiments, and safety depend on these common-sense steps. Proper handling doesn’t just avoid waste—it keeps everyone healthier, and that’s worth a few extra minutes each day.
If you spend time in a lab, factory, or even a college chemistry class, you pick up an appreciation for the safety data sheets tucked in every cabinet. People taking safety seriously saves lives, but there’s confusion around some newer chemicals on the market. 3-Ureidopropyltrimethoxysilane, a compound used to help things stick—like paints, adhesives, coatings—seems technical, but it’s a daily reality for many in manufacturing or construction.
I always look for data, not rumors, when it comes to chemical safety. According to the European Chemicals Agency (ECHA) and the U.S. National Library of Medicine, 3-Ureidopropyltrimethoxysilane can irritate skin and eyes. If you’ve gotten a splash of this stuff on bare skin, the redness and itching speak for themselves. Most reports show the effects clear up if you wash exposed areas promptly and avoid repeat contact. Data on longer-term exposure remain spotty. That lack of detail makes some people jumpy about its possible impact.
Breathing in vapors from this compound may bother the nose and throat. From my work in a research lab, I learned that strong ventilation means fewer headaches from volatile substances. Some silanes also release methanol when they react with water—too much methanol definitely causes bigger health risks like headache, nausea, and dizziness. Methanol gets more scrutiny, and rightfully so, given its reputation for toxicity if ingested or absorbed at high levels.
In practical workplaces, personal protective equipment—gloves, goggles, and a decent lab coat—brings peace of mind. Good work culture also means cleaning spills quickly and teaching proper storage habits. Containers should stay sealed. It’s not just procedure; it’s everyday protection. Most manufacturers and users respect the dangers, but things slip sometimes, so having material safety protocols visible and discussed goes a long way.
The building and automotive sectors love what this silane does for adhesion and surface treatment. Still, convenience can’t outpace thoughtful handling. Too many close calls I’ve witnessed come down to assuming a product is safe without real review. Risk comes from exposure, not just a scary-sounding chemical name. Deciding about hazard isn’t about fear—it’s more about matching facts and habits.
Toxicity often depends on dose and route of entry. It’s the classic Paracelsus lesson: The dose makes the poison. This silane’s main hazard comes from irritation, not acute toxicity at low doses. Long-term studies on inhalation or chronic low-level effects lag behind new market applications. More research would help clear the air for everyone who interacts with the product. Pressure on companies to publish toxicity data leads to better standards for all of us. No one likes mystery ingredients on the job.
We can’t let fear dictate policy, but playing fast and loose with industrial chemistry makes no sense either. If I were to offer one solution, it would be honest labeling, easy-to-read safety sheets, and company policies that expect gloves and eye wash stations, not just encourage them. Regular training and workplace conversations around chemical handling shouldn’t feel optional or rare. That’s what keeps minor irritants from turning into bigger stories.
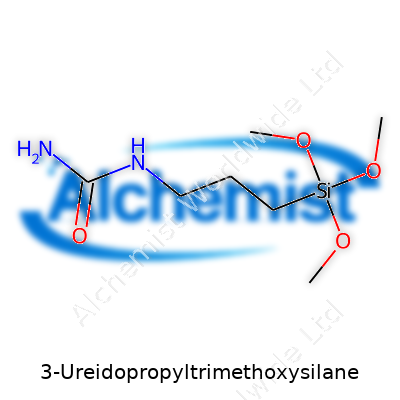
The chemical formula for 3-Ureidopropyltrimethoxysilane is C7H18N2O4Si, and it carries the CAS number 23843-64-3. In the world of organosilanes, this compound draws attention among researchers and manufacturers. My own time spent in industrial labs taught me that even straightforward additives carry weight in materials science.
Anyone who has spent time around surface treatments or adhesives will have run into silanes. They tweak how coatings stick, drive up stability, solve water resistance issues, and turn materials meant for disposal into something more valuable. The ureido group in this molecule is a real game changer, showing a special knack for bonding with both inorganic surfaces—like glass or metals—and organic polymers. This trait earns it a nod in all sorts of formulations where sticking power and durability matter.
It doesn’t just stick things together. 3-Ureidopropyltrimethoxysilane can boost a product’s long-term performance in harsh environments. Those years spent working with water-based adhesives highlighted for me that the right silane cuts down on delamination and makes shapes last longer in the real world. In construction—where anything that gets buried in concrete or exposed to wet and dry cycles faces daily stress—this is the difference between a project running for decades or falling short in a few years.
Every chemical on the market should come with clear data, and that’s where the CAS number (23843-64-3) steps in. Transparent sourcing helps buyers steer clear of counterfeits and subpar quality, which can save billions in reworks and recalls industry-wide. Even a misstep with a single bag of additive can spell disaster for an entire batch of electronics or automotive parts. I’ve seen engineers in a panic when a minor labeling mistake threatened to halt multimillion-dollar production lines.
Handling 3-Ureidopropyltrimethoxysilane isn’t a casual endeavor. The compound releases methanol as it hydrolyzes, and methanol brings both flammability and toxicity issues. Having spent time in production settings, I’ve learned the real-world importance of personal protective equipment and proper air circulation. Not every operator receives in-depth chemical training, so clear labeling—down to the CAS number—gives everyone the right cue to take it seriously.
The world uses more synthetic additives every year, raising a real question: How should companies handle hazardous byproducts like methanol, and what does responsible disposal look like? Treatment of silane-containing waste still lags behind the innovation seen on the product development side. I’ve talked to teams that took shortcuts, only to see long-term headaches from soil contamination or air quality complaints. Trust between customers, workers, and producers breaks when shortcuts enter the picture.
It helps to see collaboration between regulatory agencies, manufacturers, and recyclers. Bringing closed-loop systems and safer delivery formats should get high priority. If those of us who use or sell these chemicals speak up about the practical challenges, policy can follow technology—not lag behind it.
The chemical world brings progress, but each compound carries its own risks. 3-Ureidopropyltrimethoxysilane shows up in labs, manufacturing, and specialty coatings. It comes with hazards that call for respect and solid knowledge, not just a quick scan of the safety sheet. This compound irritates skin and eyes and can set off reactions if someone breathes in its fumes. I’ve met workers dealing with split gloves or goggles fogged beyond use— small oversights that lead to real harm. Too often, people rush or get comfy around chemicals they use often. Stories like these stick with me because, in the end, safety comes down to habits, not just labels.
Anyone managing this chemical needs to keep it off their skin and away from their eyes, and avoid breathing in vapors. A lab coat, splash goggles, and gloves— not the thin disposable kind, but butyl or nitrile— should be the norm. Ventilation changes everything. Exhaust fans or fume hoods mean you won’t face a sneaky vapor lingering longer than expected. I’ve walked into small workshops where an open window did more to protect the team than a dozen written procedures sitting in binders. Handling starts with common sense: keep containers sealed when not in use, and don’t trust your nose to spot leaks. Routine checks keep surprises out of the picture.
No one wants to clean up after a leaky drum. Proper storage means keeping 3-Ureidopropyltrimethoxysilane somewhere cool and dry, out of sunlight. It’s smart to label everything clearly. People swap containers and forget their contents. That’s how water-reactive spills happen, and how someone gets burned or worse. I’ve seen dated cardboard boxes stacked near chemicals— wrong move— since moisture brings risks. Sturdy, labeled, airtight bottles on shelves that aren’t overcrowded are the way to go.
Tossing leftover chemicals down a sink or into regular trash isn’t just bad practice— it breaks the law and puts the environment at risk. 3-Ureidopropyltrimethoxysilane doesn’t belong in waterways or with landfill waste. Disposal should go through hazardous waste programs. In my time overseeing a cleanup, ignorance often caused more issues than neglect. Folks have a duty to check local rules since disposal guidelines change by region. Coordinating with certified waste handlers isn’t just a box to tick; it keeps materials from piling up where they don’t belong.
There’s no substitute for solid training. Everyone handling this substance benefits from review sessions and real talk about near-misses or mistakes. I’ve seen workplaces hang up posters showing emergency routines, and I’ve watched new hires learn more by shadowing someone alert to risks than any video training could provide. Communities— coworkers, local agencies, and education partners— bring everyone up to speed faster than acting alone. Reporting problems early stops a minor leak or exposure from turning into a crisis. Building this sort of teamwork means no one gets left dealing with fallout alone.
Handling and disposing of chemicals like 3-Ureidopropyltrimethoxysilane isn’t just about obeying rules. It’s about watching out for each other and keeping tools, spaces, and habits in shape— day in, day out. That’s what keeps workplaces safe and landscapes clean.
| Names | |
| Preferred IUPAC name | 3-[[(Aminocarbonyl)amino]propyl]trimethoxysilane |
| Other names |
(3-Ureidopropyl)trimethoxysilane 3-(Trimethoxysilyl)propylurea |
| Pronunciation | /ˌjʊəˈraɪdoʊˌproʊpəltraɪˌmɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 23843-64-3 |
| Beilstein Reference | 1465062 |
| ChEBI | CHEBI:85345 |
| ChEMBL | CHEMBL529084 |
| ChemSpider | 20258 |
| DrugBank | DB22020 |
| ECHA InfoCard | 03c1e5fb-7c7d-40de-8dba-fb4d1792e197 |
| EC Number | 238-969-9 |
| Gmelin Reference | 146197 |
| KEGG | C18325 |
| MeSH | D017225 |
| PubChem CID | 19914047 |
| RTECS number | TP4555000 |
| UNII | YJK82M317K |
| UN number | “UN3334” |
| Properties | |
| Chemical formula | C7H18N2O4Si |
| Molar mass | 221.29 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Ammonia-like |
| Density | 1.16 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | -2.2 |
| Vapor pressure | < 0.01 hPa (20 °C) |
| Acidity (pKa) | 11.0 |
| Basicity (pKb) | 11.85 |
| Magnetic susceptibility (χ) | -62.4e-6 cm³/mol |
| Refractive index (nD) | 1.428 |
| Viscosity | 2 mPa·s |
| Dipole moment | 5.33 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 616.5 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Causes serious eye damage. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 117 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat 2000 mg/kg |
| NIOSH | NAM5412000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
3-Ureidopropyltriethoxysilane 3-Aminopropyltrimethoxysilane 3-Mercaptopropyltrimethoxysilane 3-Glycidoxypropyltrimethoxysilane 3-Chloropropyltrimethoxysilane |