
Over the years, the story behind Anilino-Methyl-Triethoxysilane (AMTES) tracks the changing needs of the chemical and material science worlds. The structure of this compound, blending organic and silicon-based building blocks, came about as industries started exploring new ways to bridge the gap between flexible polymers and rigid inorganic surfaces. Chemical pioneers searching for adhesives and coupling agents that could hold up in real-world environments stumbled onto the class of organosilanes in the mid-20th century. AMTES emerged as an answer to the limitations of earlier silanes that had trouble keeping up with the demand for tougher, smarter, and more adaptable materials. Through decades of experimentation and shifting end-user needs, this chemical has come to represent a toolkit for those who try to push the boundaries between what’s possible with plastic, glass, ceramics, and even electronic devices. Experience tells me that real progress rarely shows up as a single aha moment; it grows out of relentless trial, observation, and collaboration. AMTES stands as proof of what happens when industry listens to the needs of engineers and scientists stubborn enough to keep tinkering.
AMTES shows up as a liquid that changes the playing field for bonding and modifying surfaces. Its special appeal rests on its hybrid nature: aniline and silane groups rolled into one molecule. This combo lets it grab onto both organic and inorganic partners—a big win in the quest for better adhesion and chemical resistance. Unlike simpler silanes, which often can’t compete in harsh environments or demanding production processes, AMTES brings flexibility and chemical activity all at once. From treating glass to prepping metal and silicate minerals for composites, manufacturers turn to this molecule to close the gap between components that otherwise wouldn’t stick together. Anyone who’s watched a batch of uncured resin peel from a surface mid-test knows that picking the right coupling agent means the difference between production success and expensive recalls.
You’ll usually find AMTES as a clear to slightly yellowish liquid, sporting a mild aromatic scent reminiscent of many aniline-based materials. Its chemical formula, C12H21NO3Si, hints at its makeup: a central silicon atom tied to triethoxy groups, tethered by a methyl link to an aniline ring. In day-to-day work, this means it doesn’t mix easily with water, but grabs onto most organic solvents without fuss—making it easy to handle in the lab or on the shop floor. The boiling point sits comfortably above 200°C, so it doesn’t vanish under typical processing conditions. The triethoxysilyl end hydrolyzes in the presence of water, forming reactive silanol groups that can anchor to glass, stone, or metal oxides, while the aromatic amine handles business with polymers and resins. Its density, viscosity, and reactivity make it a go-to for anyone who needs a chemical that plays nice with both worlds.
A bottle of AMTES demands clear and informative labeling. I’ve seen more than one confusion when product identity isn’t spelled out with IUPAC names, synonyms, and batch details. Manufacturers lean heavily on lot number tracking, CAS registration, and purity statements—usually above 97%—to guarantee users know exactly what they’re working with. Technical sheets always spell out important details: flash point, specific gravity, storage recommendations, as well as hazard identification, which in the case of AMTES can center on potential skin, eye, or respiratory irritation. The best suppliers back up their specs with transparent testing and certification, including GC-MS or NMR data to chase down trace impurities that can spoil a production run. My experience tells me you’ll always pay for quality and reliable documentation up front, but it saves headaches and warranty claims down the line if something unknown sneaks into the product.
Producing AMTES starts with a careful union of methyl-aniline and triethoxysilane starting materials under controlled temperatures. The key is in guiding the hydrosilylation reaction: getting the methyl group to link with silicon while avoiding side products that can gunk up performance. Catalysts—often platinum or rhodium complexes—push the reaction along, reducing the energy needed and keeping unwanted byproducts from sneaking in. The reaction runs best in an inert atmosphere to keep out oxygen and moisture, which can sabotage the silane groups by premature hydrolysis. Purification steps can involve vacuum distillation or careful solvent extraction to pull out unwanted residues. In my experience, well-optimized batch production means less waste, less rework, and higher-quality yield, all of which keep production lines humming smoothly and final users coming back for more.
AMTES stands out for its willingness to react across a range of conditions that turn other organosilanes into wallflowers. The triethoxy groups readily hydrolyze to silanols in the presence of water or moisture, allowing them to condense and attach to silica, alumina, or glass. Meanwhile, the aromatic amine end delivers solid nucleophilicity, making it a flexible anchor point for further modifications—say, through acylation or diazotization, which opens up possibilities for even more functional materials. Mixed with epoxy, polyurethane, or other resins, AMTES often finds itself at the interface, mediating chemical bonds and boosting compatibility between otherwise stubborn phases. In surface modification projects that call for both robust chemical tying and fine-tuned surface energy, AMTES earns its keep by offering tunability without requiring exotic reaction conditions.
In the world of chemicals, names matter. AMTES is listed under several trade and chemical names, such as N-Anilino-Methyl-Triethoxysilane, N-Phenylaminomethyltriethoxysilane, and sometimes abbreviated as AMTES or APES. Supplier catalogs might have minor variations, but the CAS number ties it all together and removes confusion when buyers and sellers speak different jargon. This variety in naming typically mirrors the language or market focus—coatings, adhesives, or electronics—but beneath it all, the chemistry stands unchanged. My own work has stumbled when packaging or paperwork obscured the true identity of a key reagent, so I always double-check synonyms and synonyms for peace of mind.
Every professional who has handled AMTES or similar reagents recognizes the value of safety protocols and personal protective equipment. The main risks come from inhalation of vapors, direct skin or eye contact, and poor ventilation, which can build up exposures over time. Proper handling starts with gloves, goggles, and lab coats, and extends to fume hoods and well-labeled storage containers, kept cool, dry, and away from incompatible acids or bases. OSHA and REACH regulations require clear documentation of hazards—including MSDS sheets—with up-to-date information on toxicity, flammability, spill response, and waste disposal. I’ve learned that a small investment in training, ventilation, and emergency plans always pays off—chemical burns and respiratory incidents are avoidable with a bit of preparation and common sense vigilance.
Industries using AMTES benefit from its ability to coat and modify all sorts of surfaces, bridging differences between glass, ceramics, and a slew of polymers used in automotive, aerospace, electronic, and biomedical products. In composites, it works as a coupling agent that adds both toughness and adhesion, crucial on production lines where slip-ups can be expensive. The electronics sector takes advantage of its chemical bonding to create thin films and insulators, where clean, stable attachment determines performance of intricate circuitry. In paints and coatings, the molecule helps paints grip non-porous surfaces under challenging weather and wear conditions. I’ve watched engineers praise AMTES for its habit of turning otherwise incompatible materials into reliable, high-strength assemblies that last under real-world stresses.
Research labs and product development teams keep finding new uses for AMTES, often chasing the benefits of better durability, chemical resistance, and compatibility in demanding settings. Researchers study how subtle shifts in the chemical structure affect performance at nano and macro scales, seeking to minimize defects and maximize bond strength in high-value composites or electronics. Pilot projects continue to test its ability to stabilize nanoparticles, improve anti-corrosive coatings, or create bioactive surfaces in medical devices. Research usually involves collaboration between chemists, material scientists, and end users, with regular feedback loops pushing improvements in both synthesis and application methods. From direct lab experience, I know that product breakthroughs don’t happen without this cycle of testing, failure, and course correction—real innovation emerges from rolling up sleeves and trying new approaches.
Scientists have devoted resources to pinning down the acute and chronic impacts of AMTES on workers, consumers, and the wider environment. Studies point to primary irritation risks for skin and eyes, as well as respiratory tract effects if inhaled in confined, unventilated spaces. A few experiments extended testing to aquatic organisms, flagging the need for safe disposal to avoid waterway pollution. Ongoing toxicity profiling shapes both workplace controls and the regulatory framework, especially as new applications could alter the risk profile. Based on my observations, responsible companies never skimp on toxicity research; instead, updated findings translate rapidly into site-specific hazard assessments, personal protective standards, and even product reformulation if public health demands it. Clear communication and constant monitoring prevent problems and boost public trust.
AMTES stands poised for bigger roles as materials and electronics industries demand smarter coupling agents for new composites, hybrid structures, and next-generation devices. The push toward more sustainable, high-performance products gives a boost to molecules that increase lifespan and functional performance, which can delay waste and reduce unplanned failures in the field. Scientists and product designers study ways to tailor AMTES derivatives to work with emerging nanomaterials, 3D-printed substrates, and bioactive platforms. Improved safety and greener synthesis routes remain ongoing priorities, as global demand presses for chemicals that work wonders while also leaving smaller footprints. My experience with technology trends suggests that as applications get more demanding, the pressure grows on suppliers and researchers to keep AMTES formulations evolving—by dialling in both performance and sustainability at every stage.
Most people aren’t thinking about silane chemistry out on a morning walk. For people working in manufacturing, coatings, or advanced materials, words like Anilino-Methyl-Triethoxysilane pop up often. In my first lab job out of college, this was the bottle you’d find on the shelf next to a stack of safety goggles—the kind of thing that almost looks boring, until you realize the role it’s playing.
This molecule belongs to a class called organosilanes. The best way I can describe it: a bridge between two worlds that usually repel each other. Its tail grabs onto glass, metal, or ceramic—with the kind of grip only found in nature where barnacles stick to ship hulls. On the other end, it reaches out to organic materials: plastics, rubbers, paints, adhesives. If you’ve ever tried to glue plastic to a ceramic mug, you understand the problem. Most surfaces act like oil and water, refusing to bond. This is where Anilino-Methyl-Triethoxysilane steps in.
A lot of people assume strong adhesives just “stick.” The real magic lies in surface preparation, especially for tricky materials. My friend, who repairs wind turbine blades, swears by silane treatments—he says they turn unreliable joints into long-lasting repairs. In electronics labs, researchers use Anilino-Methyl-Triethoxysilane to coat glass slides before layering on everything from gold circuits to organic semiconductors. It anchors molecules that would otherwise float away after a rinse. Without this kind of molecular handshake, the slide gets patchy, the device fails, and the research grant doesn’t get renewed.
Industrial coatings also rely on this silane. In high-traffic areas—think airports, subway stations—paint peels unless it can truly bond to tile or steel. A quick surface wash with a diluted solution of this molecule, and the topcoat grabs on for years. The chemistry boils down to hydrolysis and condensation reactions. The triethoxysilane end reacts with water to form silanol groups, which cling to surfaces with silicon oxide links. The anilino group hangs on like a hook, grabbing whatever polymer or resin gets added next.
It’s easy to ignore where these molecules go after use. I still remember the sharp, chemical-y scent that drifted through the lab after each experiment—reminding me that good ventilation is critical. Anyone handling Anilino-Methyl-Triethoxysilane faces exposure risks. According to the National Institute for Occupational Safety and Health, organosilanes can irritate the skin, eyes, and respiratory tract. Chronic overexposure also raises long-term health questions. Chemical manufacturers and research institutions need to double down on responsible storage and handling. In my experience, labs do best with dented, well-used fume hoods and a culture that values honest reporting of spills or symptoms.
On a larger scale, there’s momentum in green chemistry circles for safer alternatives or improved disposal methods. Developing less toxic analogues or finding ways to recycle used silane solutions can lower the environmental load. The EU’s REACH regulation encourages companies to rethink chemicals that pose higher health risks. Growing consumer awareness about chemical exposure also nudges industries to make safer choices when formulating consumer products.
From aerospace composites to life-saving medical devices, Anilino-Methyl-Triethoxysilane is a linchpin for binding materials that otherwise refuse to work together. In every new smartphone display, dental implant, or flexible solar cell, there’s a good chance some version of this molecule is quietly holding things together behind the scenes. As industries keep racing ahead, smart and safer chemistry will decide which new inventions actually thrive outside the lab.
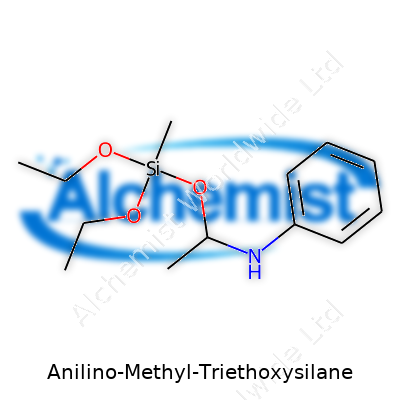
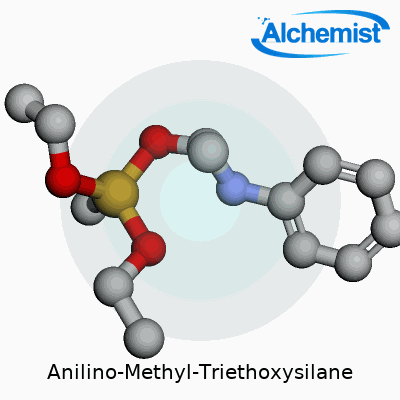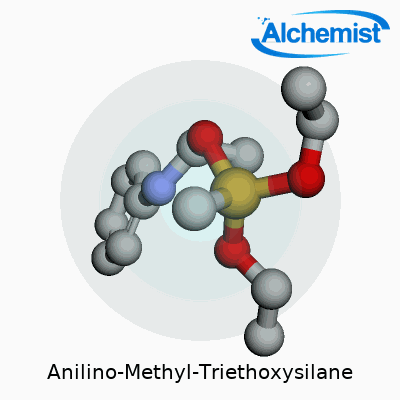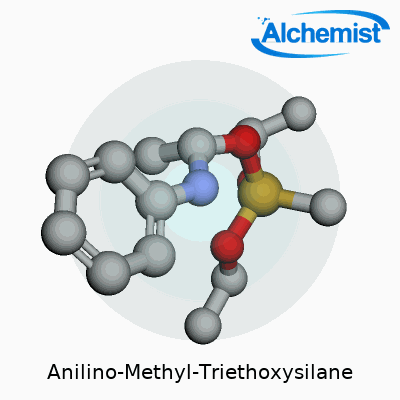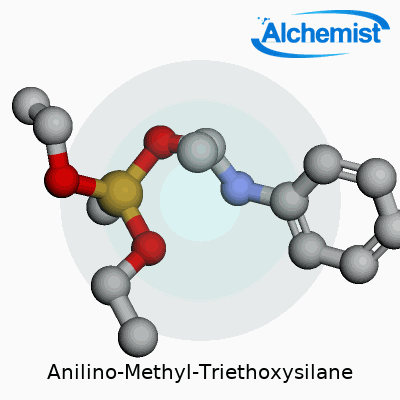
Anilino-Methyl-Triethoxysilane stands out with the chemical formula C14H25NO3Si. Written out, it reads as C6H5NHCH2Si(OC2H5)3. Here, you find a silicon atom parked at the center, snagged by three ethoxy groups and a methyl group tied to an aniline ring. This mix of silicon and organic fragments gives the compound its edge.
Anyone who’s taken on a coatings project or tinkered with composites knows that how something starts at the molecule can end up affecting how a whole product works. The triethoxysilane part helps this chemical to bond with glass, metals, and even some plastics. Surfaces transformed with silanes like this one can shrug off water, resist corrosion, and hang onto paints and adhesives years longer.
In places where electronics and optics need protection, Anilino-Methyl-Triethoxysilane finds purpose. The chemical bonds it forms create a barrier, keeping moisture and dust from sneaking in. As devices get smaller and more complex, there’s less room for error. Using the right silane helps cut down on device failures from the inside out.
Back in the early days of learning lab work, it was tempting to brush aside the details of a chemical’s structure in favor of what it did in the flask. After handling silane-based surface treatments in graduate studies, it became clear that little tweaks in molecular structure brought big changes in durability or reactivity. Anilino-Methyl-Triethoxysilane, with both aniline and silane functions, serves as a bridge between the robust world of silicon and the chemically creative side of organic molecules. That bridging effect shapes everything from automotive coatings to fiber optic cables.
Researchers and engineers rely on this chemistry to weatherproof solar panels, toughen up medical devices, and give paints the grip to resist chipping. Unlike some bulkier molecules, this formula brings enough flexibility for fine-tuned surface modifications, without the bulk that gums up production lines or warps sensitive devices.
Good results from such chemicals don’t come by accident. Handling silanes calls for solid safety practices. These compounds can react with water, producing alcohol and sometimes heat. In a busy warehouse or small lab, a stray splash can turn risky. Simple fixes—sealed containers, dry gloves, solid ventilation—cut down on those worries.
No industry moves forward on its own. Shared information about long-term effects means users can borrow from each other’s successes and mistakes. Some silanes can trigger allergic reactions or affect lungs with repeated exposure. Anyone working with Anilino-Methyl-Triethoxysilane benefits from fresh air, safety goggles, and access to current data sheets.
Better education and more transparent labeling push safer, smarter chemical use. Industry guidance already encourages careful tracking of shelf life, storage temperature, and compatibility. Following the trail from chemistry textbooks to real-world improvements, Anilino-Methyl-Triethoxysilane continues to power everything from bridges to smartphones. The real progress shows up away from the lab, in lasting performance and easier repair or recycling.
Plenty of chemicals get tossed around in labs and factories without much attention from the public. Anilino-Methyl-Triethoxysilane doesn’t exactly ring alarm bells for most people, yet talk to a chemist or safety officer and the reality looks different. This compound combines a silane backbone with an aniline group. Mix a nitrogen atom in with a silane and the story changes: aniline derivatives have always had a reputation for being risky, not just in academic papers but in dust-coated MSDS binders on site.
Even small doses of aniline can lead to health issues like methemoglobinemia, where your blood’s oxygen transport tank runs low. People exposed to vapors or spills know that odd rubbery smell, and it's not something a regular mask or open window can fix. The extra chemistry with triethoxysilane groups doesn’t remove those worries – add in the risk of flammable fumes and increased skin irritation, and now you’re holding a bottle that deserves actual respect.
Years back, I handled compounds in the same chemical family. Gloves, goggles, and fume hoods became second nature. College labs crack jokes about overprotective safety rules, but you never forget that first whiff of a solvent that makes your chest tighten. Seeing someone carelessly pipette a silane from an open bench, only to spend the night coughing, drives home why safety advice matters. Even with proper training, using something with an aniline group prompts a gut-check — each mistake can stack up and build toward those chronic health issues no one wants to talk about.
Hazardous labeling for this kind of silane isn’t just legal cover. Skin contact, inhalation, accidental ingestion – it’s all on the table if basic steps get skipped. The combination of volatile organosilicon and an organic amine jumps out on a hazardous material chart for a reason. Regulatory agencies flag these compounds not out of bureaucratic habit, but because documented research backs up both short and long-term dangers. Just because something doesn’t kill instantly doesn’t mean it’s not planting seeds for trouble down the road.
Safe handling isn’t complicated in theory: gloves made for chemical resistance, tight-sealing goggles, and a well-maintained fume hood take care of most risks. Real life throws curveballs though. A young tech on a twelve-hour shift loses focus, or heat builds up in a storage closet and bottles start sweating. Management teams never stop arguing over budgets, so corners get trimmed: “Does everyone really need new gloves every week?” Once cost-cutting takes over, the numbers behind workplace exposures climb before you realize what’s happening.
This compound sits in a gray zone where ignorance leads to real harm. Companies pushing for faster production have a duty to give staff protective gear, clear instructions, and updated safety data. Training isn’t a box to check at orientation; it grows through shared experience. Veterans passing along stories of near-misses and scars from spilled material reach young workers in ways a PowerPoint never will. Creating a culture where people speak up about unsafe practices and call out shortcuts saves lives and keeps chronic illness from becoming another sad “cost of doing business.”
Open conversations between management, workers, and health professionals make all the difference. Science gives the facts, but day-to-day vigilance keeps danger from landing on your doorstep. A careful approach doesn’t slow progress — it becomes the foundation for real innovation without paid-in-pain lessons. Every bottle on every shelf deserves respect, and the people using those chemicals are worth far more than the convenience of skipping steps.
Chemicals like Anilino-Methyl-Triethoxysilane aren’t some off-the-shelf solution you stash in the supply closet. Anyone in a lab or production floor has seen how quickly an overlooked splash, leak, or open cap can turn into a bigger problem than anyone hoped for at the start of the day. So it matters where and how you store bottles and drums of a reactive organosilane.
This compound reacts sharply with water, which means humidity, water vapor, and condensation aren’t just annoyances—they are triggers for release of ethanol and possible formation of unwanted byproducts. When I handled these chemicals, I always checked that the cap went back on tight and made sure my gloves and bench stayed bone dry. Even a sweaty hand could cause a headache.
Strong, chemical odors in the air say something’s wrong, but with Anilino-Methyl-Triethoxysilane you won’t always smell a leak in time. Setting up inside a fume hood gives instant protection and makes containment of small splashes much simpler. Even working in a well-aired space doesn’t cut it—fume hoods or local exhaust pull vapors away from anyone’s face and out of the breathing space. Goggles and gloves built to resist organics are an everyday routine, not a backup plan.
Storing this stuff on a shelf next to direct sunlight just invites vapor buildup from even mild heat. Stick with a temperature-controlled storeroom where the sun can’t get at it. Polyethylene or steel cabinets marked for corrosive or flammable chemicals do a far better job than leaving things out in the open. Labels should face outward, not hidden, to avoid fishing around for the right bottle. Let’s face it, nobody remembers every code or bottle shape during a busy shift.
Once a bottle has been opened, some folks try wrapping it in parafilm to slow down moisture intrusion. I’ve used desiccant packs and silica gel in secondary containers to mop up any extra humidity. This isn’t just good practice—left ignored, water that sneaks in causes breakdown of the silane, filling the container with ethanol and changing the contents. A ruined batch can’t be trusted, and some outfits have had to junk entire shipments after lazy closing habits.
A chemical like this isn’t forgiving if you fumble a bottle. Non-slip mats, chemical-resistant trays, and clear labelling of spill kits on nearby shelves shorten response time during accidents. In my experience, relying on memory to locate emergency supplies quickly backfires. Regular drills—turning “just in case” into muscle memory—help everyone stay on their toes. Standard procedure includes having eyewash, gloves, face shield, and first-aid within arm’s reach. Nobody wants to go hunting for goggles during a panic.
Nobody should walk into a storage room cold, expecting to wing it. Training, refreshers, and a culture of questions far outweigh an overstuffed manual nobody reads. Hazmat sheets and updated training should come out with every delivery, not just the first one. I’ve seen real safety come from people feeling confident to pause and ask before they handle unknowns, not from top-down lectures.
Taking time to get the right gear, set up safe storage, and label containers well beats any cleanup job after a mishap. It builds trust in everyone sharing the same spaces and gives peace of mind once leaving the lab or stockroom for the day.
Walk through any modern factory floor, and chances are, somewhere in that production stream, Anilino-Methyl-Triethoxysilane (AMTES) is working behind the scenes. I’ve seen it in use at a coatings plant, where it helps boost the bond between organic paints and glass or metal surfaces. It does this by acting as a connector, giving the final layer more sticking power and keeping coatings from flaking away under tough heat or salty air. Paint shops chasing tougher, longer-lasting finishes swap stories about improvements in corrosion and water resistance when AMTES enters their blend. Seeing fewer warranty returns on painted metal parts makes a strong case for this silane in automotive and architectural projects.
Anyone who has worked with glass fiber in composites knows the struggle: fibers and plastic struggle to “shake hands.” AMTES changes that. Chemically speaking, it sticks to glass on one side and forms links to plastics on the other, locking fibers inside the plastic matrix to boost strength. In my experience with boat builders and wind turbine techs, resin shrinkage and fiber slippage show up less when AMTES gets added to the stack. The end result—lighter, stiffer parts that shrug off vibration and rain—cuts costs and downtime for fleets and power operators.
Anilino-Methyl-Triethoxysilane makes a difference in the fast-evolving electronics world, too. Printed circuit boards usually need an extra layer of chemical protection to keep out moisture and dust. Coating teams use silane coupling layers to hold protective films in place—think of it as the glue layer between the silicone and circuit traces. I’ve heard from manufacturing engineers that switching to AMTES brought lower rejection rates and less frequent solder joint failures, especially under high-humidity tests. Those small improvements show up as better warranty stats in the end.
Silicones, polyurethanes, and specialty rubbers often find themselves pressed into odd shapes or harsh industrial jobs. The addition of AMTES makes these materials grip to glass, metal, or stone much more tightly. During a stint working with a team making aquarium sealants, I saw fewer customer complaints about leaks after integrating an AMTES-based primer into the recipe. Similar results pop up in the construction field, where curtain wall gaskets and door seals last longer and attach more confidently to tricky substrates.
The flexibility of AMTES can pose handling and safety questions. Hydrolysis byproducts and exposure to moisture call for tight control on the shop floor. I’ve seen smart labs invest in better humidity control for storage rooms, and supply chain managers source pre-mixed primers to give workers fewer handling headaches. Some research teams are experimenting with alternative molecules for especially eco-sensitive projects, but so far, the unique balance of chemical “bridge-building” and performance from AMTES stands tough in head-to-head tests.
From better bridges between materials to solving real reliability problems, AMTES earns its place in a busy toolbox. Broader adoption comes down to thoughtful process tweaks and ongoing safety training back in the plant. Every improvement traces back to materials scientists and techs in white coats, testing, failing, and then landing on these practical wins.
| Names | |
| Preferred IUPAC name | N-phenylmethyl-3-(triethoxysilyl)propan-1-amine |
| Other names |
3-Triethoxysilyl-N-methylaniline Anilino-methyl-triethoxysilane N-Methyl-3-(triethoxysilyl)aniline N-Methyl-3-aminophenyltriethoxysilane N-Methyl-3-(triethoxysilyl)aniline |
| Pronunciation | /əˌnɪlɪnoʊˌmɛθɪltraɪˌɛθɒksiˈsaɪleɪn/ |
| Identifiers | |
| CAS Number | 3473-76-5 |
| Beilstein Reference | 3921056 |
| ChEBI | CHEBI:33282 |
| ChEMBL | CHEMBL3636047 |
| ChemSpider | 2736052 |
| DrugBank | DB14421 |
| ECHA InfoCard | '03-2119956634-49-0000' |
| EC Number | 218-474-9 |
| Gmelin Reference | 112758 |
| KEGG | C19325 |
| MeSH | C hemicals and Drugs Category; Organosilicon Compounds; Silanes; Silanes, Aryl; Anilino-Methyl-Triethoxysilane |
| PubChem CID | 71372307 |
| RTECS number | BV6550000 |
| UNII | E4675M222Z |
| UN number | 2810 |
| Properties | |
| Chemical formula | C12H23NO3Si |
| Molar mass | 303.46 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Amine-like |
| Density | 0.99 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 1.6 |
| Vapor pressure | 0.01 hPa (20 °C) |
| Acidity (pKa) | pKa ≈ 4.6 |
| Basicity (pKb) | 5.6 |
| Magnetic susceptibility (χ) | -68 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.478 |
| Viscosity | 3 mPa.s |
| Dipole moment | 3.8744 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 334 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS05 |
| Signal word | Danger |
| Hazard statements | H302 + H312 + H332, H317, H319 |
| Precautionary statements | P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 70 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): 1780 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2200 mg/kg (rat, oral) |
| NIOSH | KRZ1750000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 3 ppm |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Aniline Methyltriethoxysilane Phenyltriethoxysilane Aminopropyltriethoxysilane Anilinoethyltriethoxysilane |