
Iso-Butyltriethoxysilane started drawing real industrial attention in the decades after the 1950s, as the organosilane chemistry field grew. Early chemists working with silicon-based compounds saw new possibilities for bridging inorganic and organic materials, and iso-butyltriethoxysilane offered a slightly branched alkyl group that could boost both compatibility and function across product lines. Firms invested in scale-up during the late 20th century, seeing this molecule’s potential in surface chemistry, especially where traditional silanes failed to deliver durable bonds in humid or variable environments. The rise of construction composites, impact-resistant glass, and specialty coatings through the 1980s all pushed demand for nuanced silanes, including this iso-butyl member of the triethoxysilane family. It reflects a steady evolution, where necessity—better adhesion and moisture-proofing—prompted real technical milestones.
Iso-Butyltriethoxysilane stands out as a colorless, clear liquid—a chemical that barely holds a scent, but quietly fills a vital role across industries. Folks handling it realize quickly that its trifecta of ethoxy groups on silicon means it can react in controlled settings to generate silanol groups, bonding to glass, minerals, metals, plastic fillers, and more. It doesn’t get shipped in fancy containers: clear solvents, UN-regulated drums, and strict labeling are the norm, reflecting how manufacturers want to balance broad market reach and safety. Only a handful of global chemical producers supply high-purity grades because stability in transport and consistency in application really matter—erring just a bit on impurities can impact polymer-based sealants or cause poor curing in resins. Not a household name, yet well-known to chemists tinkering with high-performance adhesives, construction sealers, and water-repellent treatments.
Iso-Butyltriethoxysilane has a molecular formula of C10H24O3Si and a molecular weight just under 220 g/mol. Its boiling point hovers around 180°C, and it stores well at ambient temperatures if kept dry and sealed—everyone I've met in labs carries a healthy respect for moisture intrusion here, since the ethoxy groups hydrolyze with even minimal water exposure. Its density sits below water, and it shows miscibility in alcohols and some hydrocarbons, but separates fast from aqueous solutions. That hydrolytic sensitivity gives it versatility, although unpackaged supply must always use desiccants and keep air tight to maintain product life. Color can hint at quality—best batches stay clear, with trace color indicating possible contamination or premature condensation. The reactivity isn’t aggressive unless catalyzed, letting users blend safely but also requiring knowledge for more complex formulations.
Each drum or container gets a label carrying the chemical’s name, UN number (many listings connect it to flammable organic liquids), batch code, purity percentage (typically 98%+ for industrial buyers), storage advice, and date of manufacture. MSDS (Material Safety Data Sheet) must travel with each shipment. The specs list refractive index (about 1.39–1.41), flash point above 60°C, and detailed recommendations on ambient storage—every technical manager I know double-checks this because aged or improperly handled silane can skew application results and trigger failures. Standard packaging comes in steel or HDPE drums, internally sealed, and often with a nitrogen blanket to fight off accidental hydrolysis. Nobody in regulatory compliance would sign off without GHS-compliant hazard warnings about flammability and handling.
Manufacturers typically produce iso-butyltriethoxysilane through hydrosilylation or direct alkoxylation. The starting point involves chlorosilanes, where isobutylchlorosilane meets ethanol in controlled reactors, often in the presence of an acid scavenger and under full inert gas blanket. Side-products, like byproduct ethyl chloride or HCl, require immediate removal to keep final product quality high. Plant engineers need a reliable liquid-liquid separation stage plus distillation columns, often running under reduced pressure to carefully purify the product without thermal decomposition. Batch consistency relies on tight reaction temperature and moisture control—just a trace of water in the reactor slashes yields and increases downstream purification headaches. Over time, producers refined continuous flow reactors and in-line monitoring (GC, FTIR) to raise yields and cut costs, answering real industrial demand. Many facilities have infrastructure dating back to the ‘80s or ‘90s but keep upgrading quality controls.
Iso-butyltriethoxysilane stands out thanks to its hydrolizable ethoxy groups. In typical applications, a controlled water or acidic catalyst trickle frees up silanol groups, letting the molecule bond directly to surfaces like glass, silica, metals, or minerals. That initial hydrolysis can proceed quickly, but users have to balance pH—acid speeds up things but can also create oligomers or even gels, while neutral or slightly alkaline settings slow things down. When reacting with organics, the isobutyl group gives some extra hydrophobicity to finished surfaces, which especially matters for outdoor, weatherable coatings. Chemists sometimes strip off or replace ethoxy groups for modification, but in direct use, most rely on the silane’s base structure. In more esoteric settings, downstream reactions can use iso-butyltriethoxysilane as a stepping-stone towards more functional organosilicon compounds, blending building block chemistry with focused physical property design.
Common names include Isobutylsilane triethoxy, Triethoxy(isobutyl)silane, and Silanetriol, triethoxy-, isobutyl-. Different catalogues from major producers may call it by branded trade names, but chemists and purchasing agents usually zero in on the CAS number (typically 18395-30-7) to avoid mix-ups. Sometimes suppliers label it under “alkyltriethoxysilanes,” which helps with bulk ordering across projects. Distributors catalog this under their silane coupling agents line. For specialty sealants or adhesion promoting treatments, product codes change with purity, packaging, or trace solvent content, so staying on top of supplier variation avoids confusion down the line. There are just enough close isomers out there to make double-checking paperwork a wise habit.
Direct exposure calls for proper PPE—nitrile gloves, goggles, and splash-resistant aprons. I recall several training sessions where seasoned chemical handlers reinforced that one careless splash means damaged skin or eye irritation, so full eye-wash stations and closed-system pumps are a must. Inhalation of vapors doesn’t usually present acute toxicity, but neighborhoods near production facilities still monitor emissions for longer-term exposure. The main hazard concerns link to flammability, risk of hydrolysis to ethanol (which itself brings a flammable component), and the danger posed by hydrolyzed byproducts clogging vents or pipes. Facilities emphasize dry storage, grounding for static control, and explosion-proof electricals, as these standards anchor day-to-day operations. Emergency response plans spell out steps for spills, leaks, and fire—downtime from even minor incidents can cost both time and reputation for suppliers.
Construction and composites pull the lion’s share of iso-butyltriethoxysilane, especially as a coupling agent to boost bonding between glass, metal, and polymer layers. Coatings and paints see measurable improvements in water repellency and scratch resistance when this silane goes into primer or finish coats. Cable manufacturers use it for jacketing and insulation, seeking better weather resistance, as UV, moisture, and fungus all threaten performance over long installations. Some folks in the adhesives sector bank on it to secure stubborn fillers or mineral surfaces to rubber and resin matrices. An interesting side note is its uptick in stone and concrete treatments, guarding historic buildings or monuments against freeze-thaw cycles and acidic rain. The automotive sector taps it for both coating and injection molding substrates, because modern car bodies demand lighter, more chemically resistant surfaces. These aren’t theoretical benefits: performance test data often shows measurable uplift in flexural strength, salt spray resistance, and long-term weatherability.
Labs across Asia, Europe, and North America keep searching for improvements in silane chemistry. I’ve met colleagues aiming to tweak the backbone for better solubility in non-traditional polymers or to anchor tougher, longer-lasting coatings in extreme environments, like coastal infrastructure. R&D centers run pilot programs on blends with urethanes or epoxies, chasing optimized adhesion along odd-shaped surfaces or seeking ways to lower VOCs in finished products—both for performance and regulatory wins. Some teams, especially at universities, publish in-depth studies mapping how subtle shifts in group position or chain length impact key properties. Industrial research often heads straight into field trials: bridges, wind turbine blades, and pipelines all serve as testbeds for incremental formula changes. Feedback loops matter here, as even promising new analogues face hurdles in scaling up or passing industry safety standards, so practical experience shapes the progress.
Toxicologists approach iso-butyltriethoxysilane with caution but without undue alarm, as acute oral and dermal toxicity checks in at moderate levels, and the parent compound doesn’t bioaccumulate. Chronic exposure studies show minimal carcinogenicity, though breakdown products like ethanol and silicon oxides demand separate study, especially regarding long-term inhalation risks. Lab testing with aquatic organisms points to moderate aquatic hazard ratings—spillage into waterways calls for immediate containment and cleanup. Veteran industrial hygienists stress regular air monitoring inside production and packaging halls, with focus on both raw vapor and downstream byproducts. Some research under GC-MS analysis points to issues with silicone fragment release over time, though most risks tie to incomplete curing or improper handling rather than the molecule itself. Ongoing data collection and publication—required under REACH and US EPA—drive safer process designs and better worker health protections across the industry.
The sector’s future leans on both eco-friendlier chemistry and broader market reach. Newer, greener manufacturing routes target solvent-free methods or bio-based ethanol sources to cut CO2 footprints and meet growing environmental standards. Market surveys from the past three years point to expansion in civil infrastructure retrofits, wind energy, and electric vehicle batteries, where chemical durability meets head-on with energy efficiency goals. Some cutting-edge work links isobutyltriethoxysilane to ‘smart coatings’ with self-cleaning or antimicrobial properties, aiming at both public health and lower maintenance costs. It’s inevitable that regulatory frameworks will keep tightening, especially on emissions and end-of-life handling, so companies investing in continuous toxicological review and recycling options carve out competitive advantages. The pace of change couples solid chemistry with business strategy, rewarding those who bring smart, responsible innovation to a field where function truly touches everyday life—from roads and bridges to the walls and windows around us.
Iso-Butyltriethoxysilane shows up in more places than most folks realize. People involved in sealing concrete or managing corrosion challenges might have come across this tongue-twister of a compound. To break it down, this silane plays a crucial part as a water-repellent and adhesion promoter—roles that touch everything from bridges and buildings to painted surfaces and industrial equipment.
In my years working in facilities maintenance, sealing concrete walkways often made the difference between a surface that lasted and one that crumbled after every winter freeze. Iso-Butyltriethoxysilane steps in here. Once applied to a concrete surface, it penetrates and chemically bonds to the substrate. Instead of lingering on top like a wax, it reacts with moisture and forms a hydrophobic layer inside the pores. That means water can’t sneak in, freeze, and cause cracks. I’ve watched older sidewalks water-treated with silane compounds hold out for years longer than untreated cement.
Corrosion eats up infrastructure costs, especially in factories or coastal environments where salt and moisture team up against steel. Coat a metal part with Iso-Butyltriethoxysilane, and you lay down an invisible line of defense. Here, silane acts kind of like a chemical handshake between the metal surface and anything you put on afterward—primers, paints, or coatings. It links up with the metal and with the organic coatings, so the finish hangs on tighter and moisture struggles to find a way through. That extra grip reduces flaking, and from what I’ve seen, you get fewer headaches and maintenance calls down the road.
Anyone who’s peeled off bubbling paint knows weak bonding can ruin a project. Adhesion promoters like Iso-Butyltriethoxysilane work almost like mediators—they bring together surfaces that wouldn’t usually mix. I’ve seen manufacturers add silanes to paints and sealants, especially for glass, ceramics, and plastic. Without that boost, you often get unreliable sticking and poor water resistance. With it, you get stronger, longer-lasting results without extra rework. That matters on big jobs or when dealing with harsh outdoor conditions.
Some chemicals solve problems but bring health or environmental risks that make you think twice. Iso-Butyltriethoxysilane, branded and regulated for industrial use, demands respect in handling. Safety data sheets and air handling guidance should never sit unread in a drawer. If splashed or inhaled, this compound can irritate skin and lungs. Workspaces need solid ventilation, gloves, and eye protection. Proper training and regular reviews make all the difference—I’ve worked on teams where a quick safety review before starting ensured nobody ended up in the health office.
Looking at infrastructure and manufacturing demands, silane chemistry offers protection that reduces long-term maintenance, which lowers costs and waste. By keeping water at bay and bonding stubborn surfaces together, Iso-Butyltriethoxysilane keeps plenty of roads, bridges, and products in good shape longer than they would be otherwise. Research keeps turning up safer formulas and smarter application methods, too, so health and environmental impacts keep shrinking. On-site experience backs that up; with quality chemicals and smart handling, results last and safety holds up.
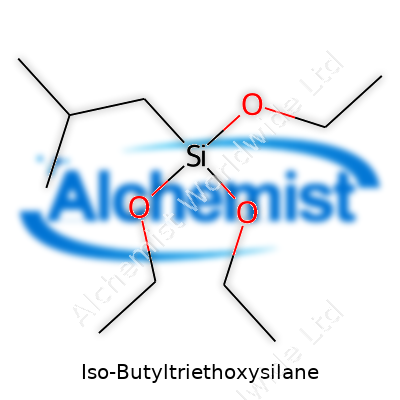
Iso-Butyltriethoxysilane might not roll off the tongue, but it's a key player in chemistry and the construction world. It sits among organosilanes, compounds that bridge the gap between organic materials and inorganic surfaces. The formula, C10H24O3Si, gives away its roots—a blend of silicon, oxygen, and an isobutyl group. This blend supports a cluster of interesting behaviors that make a real difference in practical use.
One thing about Iso-Butyltriethoxysilane—you expose it to moisture, it doesn’t just sit around. The ethoxy groups don’t resist; they react with water and release ethanol, transforming themselves into silanol groups. These new groups readily bond with mineral surfaces. In my experience, watching how quickly these changes happen under standard lab conditions gives you a real appreciation for the balance between storage and application. If you’ve ever seen glassware cloud over after an accidental drip, you’ve witnessed its stubborn tenacity. That reactivity has put Iso-Butyltriethoxysilane on the map for making durable bonds with concrete, stone, and glass.
Not all treatments for masonry and concrete are created equal. Iso-Butyltriethoxysilane soaks into porous surfaces and then forms a durable, water-repellent network. This trait helps architects and civil engineers preserve building facades and bridges from rain, freeze-thaw cycles, and salt damage. Studies back up its power: treated concrete blocks absorb up to 90% less water than untreated ones. Having worked on construction projects, I’ve seen this property extend the lifespan of infrastructure, saving costs and headaches for owners and city planners alike.
Iso-Butyltriethoxysilane carries a mild, fruity odor and a fairly low boiling point, which points to its volatility. On construction sites, this volatility sometimes causes headaches—literally and figuratively. Poor ventilation amplifies the scent, and eye or respiratory irritation can follow. The compound needs a sealed container and a cool storage spot. Anyone who’s popped a cap on a forgotten bottle will tell you: keep safety glasses nearby. The fast evaporation comes in handy for processes like spray applications, but every advantage in chemistry brings a new responsibility for safety.
Iso-Butyltriethoxysilane links the worlds of organic polymers and mineral fillers. In adhesives, sealants, and coatings, this means higher mechanical strength, improved flexibility, and longer service life. I’ve mixed it by hand into coatings and watched as the finished product stuck to stone like it grew there. Modern paints and industrial cements owe a lot to this trait, especially in outdoor uses exposed to the elements.
As construction moves toward greener practices, the environmental footprint of chemicals falls under the microscope. Iso-Butyltriethoxysilane releases ethanol during curing, but as far as organosilanes go, the byproducts count as less hazardous when compared to other coupling agents. There’s room to grow, especially as regulations tighten around volatile organic compounds (VOCs).
Research into silane chemistry keeps opening new doors for safer, longer-lasting infrastructure. Exploring blends or additives that push water repellency further without ramping up toxicity presents an ongoing challenge. Real-world durability tests—where cured concrete meets foot traffic, exhaust fumes, and winter road salts—give the final proof. Staying close to that side of things gives perspective beyond lab claims.
Iso-Butyltriethoxysilane isn’t as common as some workplace chemicals, but it’s found in paint shops, glass factories, and labs that care about moisture barriers. A few years ago, I learned the hard way how small mistakes with chemicals like this can build up. Our team had a leaky container tucked away in storage for weeks—no one paid much attention. Suddenly, fumes started drifting into our break room. We ended up pulling the fire alarm and calling HAZMAT because nobody could figure out what had caused the headache and burning eyes. Most chemicals won’t announce themselves until after they’ve settled into walls and lungs.
Iso-Butyltriethoxysilane reacts with water vapor in the air, forming silanols and ethanol. This reaction might sound harmless on paper, but ethanol vapors build up fast and both the vapor and the chemical itself catch fire easily. A splash can irritate skin and eyes right away. A sealed, labeled metal drum can stop most trouble before it starts. If you’ve got a warehouse with open skylights, forget about keeping these drums in the same space as cleaning equipment or food.
Chemical storage doesn’t get much simpler than a cool, dry spot away from sun and heat. Stacking heavy drums on a shaky wooden shelf turned out badly for one of our partner sites. One drum cracked at the edge, and getting the residue off the floor and gear took hours. A locked chemical cabinet with strong metal shelving and no water pipes overhead kept things easier. Pouring the compound always requires a proper face shield, chemical gloves, and a splash apron. More than once, folks reached in with basic gloves and ended up with red, itchy hands—so the switch to thick nitrile gloves made a huge difference.
I always insist that new colleagues review the latest safety data sheet before pouring or mixing. This isn’t just red tape. Storage rules never come from thin air. The work of agencies like OSHA and the European Chemicals Agency builds on thousands of real-world mishaps. Policies are not just for peace of mind; they trace a line from old accidents to safer habits.
Many smaller facilities don’t bother installing air handling systems built for chemical vapors. Fans that point right out a window just scatter fumes into the parking lot or the next room. Installing a real vent system with regular maintenance keeps indoor air safe. Training gets overlooked, too. Teaching a new crew once at orientation doesn’t cut it. Regular drills and “what if” discussions make sure everyone reacts fast, not frozen, when a spill happens. The best labs invite questions, share stories of close calls, and remind everyone that locked cabinets and safety showers aren’t for show.
Few industries can afford the cost of shutting down to fix chemical mistakes. Planning and daily vigilance go further than expensive tech ever could. Keeping track of container conditions and storage logs sets a strong example. My crew holds each other accountable, checking labels and containers together. Over time, this turns safety from a checklist into a daily instinct. By being honest about the risks and using our combined experience, everyone heads home with all ten fingers and a clear conscience.
Iso-Butyltriethoxysilane shows up in products many folks use. Construction workers, chemical plants, even folks mixing concrete sealers can run into this compound. It's not a chemical that stays tucked away in a lab. From my time around warehouses and industrial cleaning, I’ve learned to pay close attention to the safety data on any clear liquid with a name this long.
Breathing in the fumes from Iso-Butyltriethoxysilane causes problems fast. You don’t have to look far for real cases: headaches, nausea, sometimes burns on the skin where the chemical spills. Eyes sting if you get a splash. OSHA lists similar silanes as irritating to both skin and eyes. The main warning sign for me is always the ease with which vapors travel, especially in poorly ventilated spots. The liquid gives off enough vapor to make you feel sick if you aren’t careful.
There’s also a fire risk. Iso-Butyltriethoxysilane ignites pretty easily, and the stuff burns with a barely visible flame. I once saw a can go up in a supply room, not much warning at all, which can be the difference between putting out a spark and calling the fire department.
Federal safety offices recommend full gloves, splash goggles, and good airflow—simple measures but often skipped until someone gets hurt. The material safety data sheets lay it out clearly. You don’t need to absorb a wheelbarrow’s worth; a splash or a day breathing the vapors leaves an impact. Repeated exposure leads to chronic issues, with some people developing allergies or asthma-like symptoms over months.
I checked the EPA’s database and did not find Iso-Butyltriethoxysilane listed as a carcinogen, but the absence of proof doesn’t always mean proof of absence. Toxicity studies often lag behind real-world exposure, so treating new or under-studied compounds with respect always feels like the smart move.
Pallets arrive damaged, lids don’t always close well, and busy shifts push people to ignore smells or minor splashes. I can remember the sticky feeling after handling silane-based sealers with thin gloves—not worth the gamble. Many companies store drums near heat sources, or mix chemicals in rooms without proper airflow, making vapors harder to avoid.
For workers, the long-term risk isn’t just about one incident; it's the little exposures day after day. Handling silanes without gear, even for short periods, adds up. Managers who don’t understand the science might skip proper training, and you end up with a cycle of preventable injuries.
Keeping people safer around chemicals like Iso-Butyltriethoxysilane means upfront training. I’ve pushed for labels in plain language, not just codes. New hires should get proper gear on day one, not after an accident. If someone feels dizzy in a shop with open drums, solve the airflow issue before anything else.
Big plants and small contractors both do better with regular reminders. Replace leaky drums, use proper containers, demand masks and gloves. These habits have kept incidents off the record in shops I’ve worked at. It’s not about living in fear; it’s about not letting convenience top safety. Just a hint of chemical smell should move someone to check the ventilation and grab the right gloves.
Iso-Butyltriethoxysilane seems like a mouthful, and I’ve seen more than a few folks’ eyes glaze over when they run into names like this. Yet, beneath that tongue-twister, this compound sits behind some everyday products and industries that shape a lot of what we touch, drive, and live in.
Here’s where Iso-Butyltriethoxysilane plays a sturdy role. In the construction world, water causes headaches — concrete, stone, and bricks all break down quicker when moisture gets in. Over the last decade, research has shown that silane-based treatments can slash water absorption in concrete by up to 85%. Iso-Butyltriethoxysilane creates a barrier inside the pores of materials like concrete and masonry, locking out water while letting the structure breathe. I’ve talked with contractors who swear by these products for new bridges and walkways, especially in places with freezing winters and lots of rain. Keeping that water out extends the lifespan of public infrastructure and cuts down on repair costs, which, at the end of the day, means fewer potholes and safer surfaces.
Car makers have pressed Iso-Butyltriethoxysilane into service on a few fronts. Tire compound manufacturers want their rubber to stick well to metal wires and fabric. Adding this silane to the mix gives tires a stronger bond, leading to fewer blowouts and longer tire life. Parts exposed to weather — like exterior trims, seals, and gaskets — benefit from the same kind of resistance that keeps concrete dry. I’ve spoken with automotive engineers who look for reliability over flashy innovation. For them, using this chemical means fewer warranty claims and happier drivers down the road.
Years ago, I watched a house painter grumble about peeling paint after just one season of rain. The solution? Formulators turned to silanes like Iso-Butyltriethoxysilane for improving how paint binds to mineral surfaces — especially tricky ones like old bricks or stucco. It helps paint and coatings grip tightly, meaning finishes don’t flake off after the first storm. Paint chemists have published results showing improved durability and less water penetration. This has real implications: Better coatings mean homeowners and businesses can skip expensive rework, and cities can keep public buildings looking fresh longer.
Water and electronics don’t mix. Even small amounts of moisture can wreck sensitive boards and chips. In recent years, several electronics companies have used Iso-Butyltriethoxysilane as part of moisture-proofing strategies for semiconductors and housing materials. This has helped keep gadgets safe from accidents, which matters as everyone relies more on technology every year.
Cost stands as a real issue for many smaller operations. Silanes carry a price tag that can scare off smaller builders or manufacturers. One possible fix involves better outreach and education. Many builders and tech teams simply miss the memo on these compounds because they’re busy or skeptical. Sharing case studies that show how Iso-Butyltriethoxysilane prevents failures could move things forward. Industry groups, universities, and suppliers all have a piece to play in making useful chemical advances part of everyday work.
After years of watching construction crews and manufacturing plants try to keep pace with wear and weather, it’s clear that smart chemistry improves daily life in ways we often overlook. Iso-Butyltriethoxysilane, complicated name and all, earns its place across industries by solving problems that affect everyone.
| Names | |
| Preferred IUPAC name | triethoxy(2-methylpropyl)silane |
| Other names |
Triethoxy(2-methylpropyl)silane Isobutyltriethoxysilane Triethoxy(isobutyl)silane Silane, triethoxy(isobutyl)- |
| Pronunciation | /ˌaɪsoʊˌbjuːtlˌtraɪˌɛθɒk.siˈleɪn/ |
| Identifiers | |
| CAS Number | 18395-30-7 |
| Beilstein Reference | 3529880 |
| ChEBI | CHEBI:87154 |
| ChEMBL | CHEMBL185167 |
| ChemSpider | 21968620 |
| DrugBank | DB11242 |
| ECHA InfoCard | 100.096.777 |
| EC Number | 206-971-3 |
| Gmelin Reference | 110211 |
| KEGG | C18553 |
| MeSH | D016355 |
| PubChem CID | 10479 |
| RTECS number | WZ1800000 |
| UNII | E2F20J1D1M |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID2021507 |
| Properties | |
| Chemical formula | C10H24O3Si |
| Molar mass | 234.39 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Alcohol-like |
| Density | 0.875 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 1.94 |
| Vapor pressure | 0.3 hPa (20 °C) |
| Acidity (pKa) | 11.4 |
| Basicity (pKb) | pKb: 6.0 |
| Refractive index (nD) | 1.3900 |
| Viscosity | 1 mPa.s at 25°C |
| Dipole moment | 1.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 568 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -8131.3 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 64 °C |
| Autoignition temperature | 230 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): > 2000 mg/kg (rat, oral) |
| NIOSH | K1775 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 5 ppm |
| IDLH (Immediate danger) | NE (Not Established) |
| Related compounds | |
| Related compounds |
Trimethoxyisobutylsilane Triethoxyethylsilane n-Butyltriethoxysilane Triethoxyvinylsilane Phenyltriethoxysilane |