
Chemists started looking at organosilanes like N,N-Dimethylaminopropyltrimethoxysilane back in the mid-twentieth century, hunting for ways to bridge organic and inorganic chemistry. Industry needed something that stuck better to glass, ceramics, and metals. Over decades, work in research labs pushed the boundaries of surface treatment—first using basic silane coupling agents and then fine-tuning them for specific functionalities. N,N-Dimethylaminopropyltrimethoxysilane rose to attention thanks to its unique combination: a trimethoxysilane group for strong bonding to mineral surfaces, and a tertiary amine that interacts with resins and polymers. This push for tougher, longer-lasting composites in everything from electronics to water-repellent coatings brought the compound out of academic journals and into industrial supply chains.
Plenty of chemists know N,N-Dimethylaminopropyltrimethoxysilane under several names: DMAPTMS, 3-(Dimethylamino)propyltrimethoxysilane, or Silane A-2310 in trade catalogs. No matter what you call it, the chemical connects silane technology and organic chemistry. Its defining feature is the trimethoxysilane group, which hydrolyzes easily when exposed to water, latching onto hydroxyl-rich surfaces like glass or silica. The propyl bridge holds the dimethylamino group, opening doors to crosslinking and compatibility with numerous organic polymers. With these properties, the product finds itself in adhesives, surface modifiers, paint additives, and high-performance sealants. Most people in R&D talk about it as a workhorse—reliable, with predictable changes to surface energy and adhesion.
In its pure form, the compound presents as a colorless to pale yellow liquid, with a slight amine odor that lingers around the flask if you’ve ever uncorked a fresh bottle in the lab. The molecular formula, C10H25NO3Si, hints at a volatile material; boiling point hovers around 210°C, and it carries a density of about 0.95 g/mL. The compound doesn’t care much for water until it reacts—and react it does, rapidly hydrolyzing to form silanols, which love to cross-link on a surface. The dimethylamino group isn’t just along for the ride; it’s basic, which alters how the compound performs in acidic or basic environments. This dual nature means bottles don’t last forever—careless storage with moisture leads to gelation and loss of function.
Chemists and procurement managers checking the label expect a minimum purity level (98% for most high-grade sources), a moisture limit (often under 0.1%), and a refractive index value as a quick fingerprint for quality assurance. Shipping labels warn of flammability and moisture sensitivity. I’ve seen companies print clear guidance on shelf life and forbidden combinations, like strong acids or oxidizers, to avoid mishaps in storage rooms. Packaging uses amber glass or steel to keep light and moisture at bay. Many technical sheets also spell out regulations—the European REACH and American TSCA registrations—as proof of compliance and traceability, easing worries around restricted substances or workplace safety.
The industrial route to N,N-Dimethylaminopropyltrimethoxysilane usually relies on hydrosilylation. Producers bring together allyldimethylamine and trimethoxysilane in the presence of a precious metal catalyst, often platinum-based, under an inert atmosphere to block moisture and air. This reaction joins the trimethoxysilane to the propylamine with high selectivity. The post-reaction workup strips out unreacted starting materials, purifies the product by distillation, and bottles it under nitrogen or dry air. Smaller specialty batches might use alternate routes, but hydrosilylation dominates because of its scalability and reliability. Mistakes in moisture control lead to gel formation—a headache that plagues poorly managed operations.
Most action with this silane takes place on surfaces. Hydrolysis turns the methoxy groups into silanols, which build covalent links with glass, quartz, or metal oxides. Crosslinking forms robust, water-resistant bonds. Chemists leverage the amino functionality on the organic end for reactions with isocyanates in polyurethane systems, epoxides in coatings, or even aldehydes for surface grafting. Certain processes quaternize the dimethylamino group, creating permanent cationic centers for specialty coatings or as compatibilizers in polymer blends. The versatility flows from this unique combination: the silane anchors to the surface; the amino group delivers chemical flexibility rare among other alkoxysilanes. I’ve seen teams use this approach to modify filler surfaces in composites, nailing down dispersion and improving mechanical properties by double-digit percentages.
Anyone ordering this chemical must learn its aliases: 3-(Dimethylamino)propyltrimethoxysilane, DMAPTMS, A-2310, Silquest A-1110. Some catalogs drop the numbers in favor of structure, calling it Trimethoxy(3-(dimethylamino)propyl)silane. Regulatory filings may list EC numbers (216-846-1) or CAS numbers (2530-86-1)—both keep supply chains straight as products move internationally. Synonyms vary less in practice, but checking the technical datasheet always saves an embarrassing reorder or compatibility issue.
Lab safety officers insist on training before working with N,N-Dimethylaminopropyltrimethoxysilane. Even after decades on the shelf, this chemical presents real hazards: skin and eye irritation, vapor inhalation, and exothermic hydrolysis. I’ve learned to turn on local exhaust ventilation and suit up with nitrile gloves, goggles, and lab coats before handling. Spillage reacts fast with air moisture, so containment and cleanup procedures rely on absorbents and proper disposal—never down the drain. Chemical Hygiene Plans at larger facilities include permanganate testing for vapors, routine monitoring, and strict segregation from acids and oxidizers to prevent fires or unwanted reactions. Safety Data Sheets demand attention, stating clear guidance for both routine use and emergency response. Large-scale operators commit to annual training refreshers and deploy automatic detection to track leaks and exposures.
Real-world use stretches from construction to electronics. In the adhesives sector, manufacturers rely on this silane to bond difficult substrates: plastics to glass, metals to composites, even nonstick surfaces to paints. The electronics industry counts on thin silane films to alter dielectric properties in circuit boards or act as adhesion promoters in encapsulants. Water-repellent treatments for masonry or textiles lean on the covalent bonding between silanol and mineral surface—coats last longer and resist alkali attack. Paint formulators deploy it as a dispersing agent, making pigments easier to blend and improving weathering resistance. These applications show up far from the lab—on bridges, in smartphones, under the hood of your car. The amine group’s reactivity also entices researchers designing antimicrobial coatings or exploring new flame retardant approaches. As demands for stronger, more durable materials keep rising, the value of this silane grows.
Research teams continuously chase ways to modify surface chemistry for better product performance. My experience in an industrial R&D setting taught me that subtle tweaks—like changing the alkyl chain or swapping amines—deliver surprising gains in mechanical strength, water resistance, or compatibility with new matrix resins. Some researchers work on green chemistry approaches, seeking catalysts that exclude heavy metals, or low-energy synthesis routes that shrink the carbon footprint. Interdisciplinary projects blend materials science and analytical chemistry, tracking how each nanoscale modification impacts finished goods. I’ve seen a few startups build surface modification “toolkits” based on this silane and its cousins, proving the market hunger for customizable surface chemistry. The intersection of silane chemistry and polymer science is ripe for innovation, drawing on decades of data but still loaded with unsolved challenges.
Most published studies mark N,N-Dimethylaminopropyltrimethoxysilane as a low-acute-toxicity substance if handled with care, but the story doesn’t stop with acute effects. Chronic exposure and potential bioaccumulation remain open questions, prompting more stringent workplace monitoring. Animal studies display mild to moderate inflammation at high doses; no evidence marks it as a carcinogen, but repeated skin exposure in lab animals triggers irritation. Respiratory exposure—thanks to volatile amines—gets flagged in studies examining occupational risk. Regulatory agencies call for closed systems and local exhaust, along with medical surveillance in large users. Labs working on alternatives have begun benchmarking toxicity profiles against newer “green” silanes, pushing for materials with lower health impacts and easier biodegradability in case of release. Toxicologists routinely publish updates, responding to both industry needs and mounting regulatory pressure.
Looking forward, the roles for N,N-Dimethylaminopropyltrimethoxysilane keep growing. Sustainability pressures drive chemical engineers to retool processes, exploring broader applications in eco-friendly coatings, waterborne adhesives, and recyclable composites. The surface chemistry toolkit evolves as more companies demand solutions for renewable energy—silane-modified glass panels in solar, tough adhesives for wind turbine blades, and new insulation materials in green buildings. All these demands require adhesion, crosslinking, or chemical modification that only versatile agents like this one deliver. Digital manufacturing and rapid prototyping use surface-modified fillers and resins for 3D printing, signaling fresh opportunities. Regulatory shifts might force reformulation, but ongoing R&D, health studies, and industry feedback keep shaping best practices. With enough momentum, the next generation of materials could leave a lighter footprint, perform better, and last longer. The work goes on, and the lessons from decades of hands-on experience guide both cautious adoption and the next wave of invention.
Ask anyone who’s worked with adhesives or coatings, and the subject of “silane coupling agents” comes up quickly. N,N-Dimethylaminopropyltrimethoxysilane stands out in this group. Its primary use comes from its ability to act as a bridge: it links materials that otherwise refuse to mix. You see this magic on a production floor that’s coating glass fiber with resin or when manufacturers try to get a plastic surface to bond with metals or ceramics.
My first encounter with this compound came years ago during a project with composite materials. If you try to bond glass fibers into a polyester resin, it often feels like wrangling two unwilling dance partners. N,N-Dimethylaminopropyltrimethoxysilane changes that. One end of its molecule latches onto the glass, while the other end grabs the organic resin. The bond holds strong, not just under normal use, but through stress, heat, and time.
A 2022 market research report from MarketsandMarkets points to the growing demand for silane coupling agents, especially in the automotive and construction industries. People want cars that stay lighter but tougher, bridges that shrug off winter’s punishment, buildings that don’t peel or crumble. Coupling agents like this one help companies reach these targets.
Plenty of industries chase after better adhesion, but the impact of this compound goes even further. Take sealants and paints. Adding N,N-Dimethylaminopropyltrimethoxysilane helps paint hold onto surfaces that usually reject it, like plastics or metals. This isn’t just about cosmetics—the difference can mean the paint resists water, lasts longer, and protects what’s under it.
If you walk through a modern concrete production plant, you may also see silanes at work. They treat concrete to keep water from seeping into tiny pores, which can cause cracks or even bigger failures a few winters down the road. According to the American Concrete Institute, surface treatments like these add years to the life of infrastructure without much extra cost.
There’s a trade-off for this performance. In my experience, handling silanes—including this one—demands good training. The compound reacts with moisture and gives off methanol, which isn’t something to take lightly. Strong ventilation, gloves, and eye protection are the minimum for a safe workplace. Chemical manufacturers share clear handling sheets, and it pays to read them closely.
Waste management presents another hurdle. Companies that use N,N-Dimethylaminopropyltrimethoxysilane need solid waste plans. Most public environmental records show it’s manageable if plant staff stay disciplined. Regular training and updated protocols protect both workers and the surrounding community.
Science keeps pressing forward. Research from groups like the American Chemical Society shows ongoing progress in developing new silanes that deliver higher performance with lower hazards. Until the next generation of products shows up, the right training and strong safety culture let us get all the benefits from compounds like this without cutting corners.
N,N-Dimethylaminopropyltrimethoxysilane isn’t the kind of chemical most people bump into every day, but the impact runs through industries that touch all our lives—from sturdier cars to longer-lasting roads and better housing materials. Smart manufacturing uses it not just for adhesion, but as a tool for stronger, safer, and longer-lasting products.
Mixing up silane coupling agents can get complicated fast, especially if someone’s trying to squeeze better results out of tired old formulations. N,N-Dimethylaminopropyltrimethoxysilane grabs plenty of attention because it brings a secondary amine group into a world typically ruled by simple alkoxysilanes. Its structure lets chemists reach for a bit more control when building up adhesion between substrates and resins, whether it’s for plastics, glass fibers, or mineral fillers.
There’s a reason people ask if it plays nice with others: compatibility in these mixtures can mean the difference between killer performance and a sticky mess. Not every silane enjoys sharing space, thanks to the variety in functional groups and reactivity speeds. In the laboratory, I’ve seen premixed silane blends turn cloudy overnight, and that’s a real problem if someone’s aiming for shelf-stable additives.
From experience, trial mixes with N,N-dimethylaminopropyltrimethoxysilane and epoxy or vinyl silanes work out as long as you keep moisture out and mix immediately before use. Alkoxy groups will start hydrolyzing if water sneaks into the blend, kicking off condensation reactions that leave unwanted particles floating in solution. These side reactions can wreck the point of using a silane in the first place.
The amine group in N,N-dimethylaminopropyltrimethoxysilane sets it apart. Paired with acidic silanes, like those with methacryloxy or chloropropyl groups, things can get dicey. Acid–base reactions pop up, and the resulting byproducts can shift the final surface chemistry in unpredictable directions. This might mean weaker bonds at the interface or poor wetting of one substrate.
Some companies try tossing everything together for cost-saving blends. That approach feels risky unless you’re testing every batch for changes in viscosity, pH, and dispersion quality. Without a tight grip on quality control, you wind up guessing rather than engineering solid performance. Industry reports show that success usually comes when each silane gets added at its own stage — not from melting-pot premixes.
Over the years, I’ve watched production lines slow down because an incompatible silane combo led to gelling, clogging up pumps and pipes. Even when it seems fine at first, shelf life gets shorter, costing suppliers more in the long run due to returns and rework. Dow, Wacker, and Momentive have published warnings about mixing highly reactive aminosilanes with other silanes except for fresh, on-site blends.
It’s not all roadblocks. Some customers find that using N,N-dimethylaminopropyltrimethoxysilane as a primer layer and topping it off with another silane works better than blending from the start. Treating surfaces in stages lets each layer do its job without cross-reacting with its neighbor.
Nobody wants to dump time or raw materials chasing reactions they can’t reproduce. Testing for single-use cases, using fresh solutions, and never assuming two silanes can stay stable together are hard-won lessons. The biggest breakthroughs happen in small, carefully controlled batches, not in a one-size-fits-all drum. Sticking to verified compatible blends keeps the process reliable and finished products strong.
Mixing N,N-dimethylaminopropyltrimethoxysilane with others isn’t out of reach, but it calls for good lab work, honest tracking, and some real caution. That’s what pushes manufacturing to be more responsible with chemistry, and in the long run, it’s what gives the best performance on the factory floor.
Every time I’ve worked with specialty chemicals, one point has always stuck out: storage shapes not just the safety of the space, but the product’s real performance. N,N-Dimethylaminopropyltrimethoxysilane (DMAPTMS) isn’t as forgiving as some simpler solvents. It’s a silane, which means it will eat up moisture in the air and start reacting if left open or kept somewhere humid. That creates a mess on shelves and ruins its usefulness in bonding or surface treatment work.
Storing DMAPTMS in a tightly sealed container is the start. Every chemist who has pulled a crusty, half-hydrolyzed bottle from a shelf knows those white flakes signal both waste and problems. It pays off to keep the original container tightly closed, using a screw cap or secure fitting—some people add a desiccant pouch, which offers cheap insurance.
Sunlight causes more than faded labels. With organosilanes, direct light and heat change the way these molecules behave, sometimes degrading them and sometimes pushing them to react faster than they should. DMAPTMS belongs in a cool, shaded spot. Temperatures between 2°C and 8°C—the kind found in a chemical refrigerator—help keep it stable for months, even years in some labs. Just don’t freeze it, as low temperatures past freezing can also mess with the compound.
The fumes that rise from a bottle of DMAPTMS don’t just smell bad—they can irritate eyes and skin. Anyone storing this material should pick a well-ventilated space. A chemical storage cabinet with vents makes sense, especially in busy labs. I’ve worked in spaces where a simple shelf near an open window felt good enough, but risk and cost stack up fast if vapors linger or bottles tip over.
Mixing incompatible materials in one drawer or fridge tempted me in my earliest lab days, but it only takes one accident—like water from a leaking bottle touching a silane—to teach a lesson. Keep DMAPTMS away from acids, strong oxidizers, or water sources. Any exposure spells trouble, from fire hazards to ruined stocks.
I’ve faced audits where poor labeling on chemicals, even ones used every day, landed the lab in hot water. It’s best to label every DMAPTMS container with receipt and opening dates. Periodic checks on liquid state and labeling stop confusion when people rotate or leave the team. Some folks set calendar reminders to review their chemical stores every season.
DMAPTMS isn’t cheap, and chemical waste fees hit hard. Following all safety datasheet suggestions keeps things simple: sealed containers, cool rooms, dry spots, and good signage. If a bottle outlives use or looks off, waste it safely rather than chancing a failed experiment or a health scare. Labs following these routine steps avoid surprises and keep their budgets in check.
Getting storage right doesn’t just help the bottom line. It keeps the science honest, the people safe, and the workplace running smooth.
Walking into a lab with a bottle of N,N-Dimethylaminopropyltrimethoxysilane on your bench isn’t quite like dealing with salt or sugar. It’s a clear liquid, but don’t let looks fool you. Most people don’t want it on their skin and definitely not in their lungs. A good number of silanes, including this one, cause strong irritation—in some cases, even chemical burns. Once, trying to clean up a drip without gloves led to red, itchy skin for days, and I had to explain to a supervisor why I needed to leave early for the doctor.
Folks who work with this chemical use gloves, lab coats, and safety goggles every single time. Latex or nitrile gloves work, but swapping them if you get a splash is vital. One coworker thought her gloves would hold up all afternoon; they didn’t. After a leak, her hand stung for hours. I always keep a fresh backup pair close, and I recommend others do the same.
Eye protection is non-negotiable. The vapors cause eye irritation, but a splash is worse—think burning pain and a trip to the eyewash station. People in these labs take off jewelry and watches, roll down their sleeves, and keep goggles on—even when pipetting small amounts.
Working in a fume hood is less about following a silly rule and more about avoiding health risks. Fume hoods pull vapors away from your face and get rid of the smell, which is sharp and not pleasant. A lab that skimps on ventilation usually sees more complaints about headaches and burning eyes. In one case, we had to evacuate for an hour when someone let too much evaporate. It’s better to take the extra step to handle it under proper ventilation.
Pouring straight from a large container doesn’t save time. It risks spills and overexposure, especially in summer heat when vapors rise fast. People use secondary containers and pour slowly. Absorbent pads sit under syringes or pipettes to trap drips. After each use, wiping down surfaces cuts down on buildup.
Not every chemical needs its own cabinet, but this one goes in a cool, dry spot. Humidity breaks down silanes, and leaking bottles can corrode shelving. Folks check containers for cracks and make sure lids fit tight. If a bottle tips, the correct action isn’t to grab some paper towels—it’s to sprinkle on a spill control powder, let it soak, and scoop it up with gloves on. You never want wet silane soaking into your skin or the floor.
Lab teams run yearly safety drills for handling chemical exposures. Most emergencies involve splashes or inhaling vapors. Quick response means rinsing eyes or skin with water for 15 minutes, then getting checked by occupational health. No one complains about the interruption, since everyone’s heard stories about burns getting worse if you wait, especially with silanes.
Sharing stories helps new folks learn. Safety practices stick when workers pass down real consequences—the sting of exposure or the panic of a poorly contained spill. Mistakes push everyone to tighten up routines. Even veteran chemists double-check their safety steps because shortcuts lead to trouble.
Consistent, no-nonsense practices make handling N,N-Dimethylaminopropyltrimethoxysilane a routine, not a risk. Talking about what happens when safety steps get skipped impresses the lesson deeper than any poster on the wall. At the end of the day, everyone wants to go home without burns or ruined gear. That’s what counts most.
N,N-Dimethylaminopropyltrimethoxysilane, or DMAPTMS for short, shows up in plenty of formulations. You see it in adhesives, coatings, and textile treatments. As a silane coupling agent, it holds promise for connecting the world of organics and inorganics—plastic to glass, resin to metal. People often look for ways to use silanes in water-based systems because water costs less and is friendlier to the environment than many solvents.
Mixing DMAPTMS with water means walking into some sharply reactive territory. Silanes react with water pretty quickly. Those three methoxy groups attached to the silicon atom start to hydrolyze. They turn into silanols and methanol, kicking off a domino effect. If you let the solution sit, the silanol groups start linking with one another, building polysiloxane chains. These linked chains can fall out of solution as a gel or solid. Left unchecked, this process not only wastes material but ruins the performance of your end product.
People working in formulations don’t just accept these challenges. They develop workarounds to take advantage of DMAPTMS’s reactivity without losing control. One method hinges on strict pH control. Keeping the water on the acidic side, somewhere between pH 4 and 5, slows down hydrolysis and condensation. Buffering agents like acetic acid make it easier to manage the silane’s life span in solution.
Adding DMAPTMS to water just before use keeps things as fresh as possible. Every time I’ve seen a team try to store these solutions for days, the same story unfolds—the silane gels up, equipment needs cleaning, and there’s a chunk of wasted material. By blending just before application, you take advantage of the silane’s surface-bonding action before gelation can get in the way.
Safety and regulatory folks keep a close eye on the byproducts. Methanol isn’t just a harmless bonus; it’s toxic. Factories need ventilation and proper handling protocols to keep workers safe and waste within regulated limits.
From a quality control angle, stable performance matters. If a water-based coating turns gummy during storage, it sends a bad signal to both the production team and the customer. Engineers want treatments to work the same every time, not just once under perfect lab conditions. This pushes the industry to tweak storage, mixing, and application guidelines constantly.
Not every project jumps back to organic solvents just because silanes act up with water. I’ve seen promising attempts to microencapsulate silanes, storing them inside water-dispersible microspheres. These capsules rupture during application, keeping reactivity high but lengthening shelf life and stability. Some researchers turn toward co-solvents—mixing a little alcohol in with water keeps silanes happier for longer.
Formulators also play with loading levels, less silane in the water means less chance for runaway reactions. Sometimes, additives stabilize the solution, holding silanol groups apart long enough to reach the substrate in one piece.
The stakes aren’t just technical. Taking DMAPTMS into water-based systems brings process safety, environmental impact, and material efficiency to the front of the conversation. Each workaround and new method bumps up against expectations for safer, greener products. Success stories ripple across industries—from windshield adhesives that last longer, to carpet treatments that leave fewer residues in homes.
As more industries look to phase out harsh solvents, the challenge grows. DMAPTMS in water doesn’t give an easy ride, but it pushes chemists and engineers to rethink the basics and chase smarter solutions.
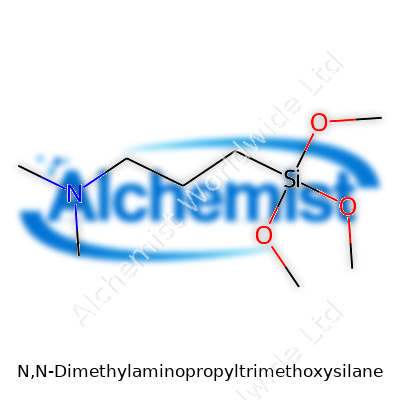
| Names | |
| Preferred IUPAC name | 3-(Dimethylamino)propyl(trimethoxy)silane |
| Other names |
3-(Dimethylamino)propyltrimethoxysilane Trimethoxy[3-(dimethylamino)propyl]silane N-[3-(Trimethoxysilyl)propyl]-N,N-dimethylamine |
| Pronunciation | /ˌɛnˌɛn daɪˌmɛθɪlˌæmɪnoʊ ˌproʊpɪl traɪˌmɛθɒksi saɪˈleɪn/ |
| Identifiers | |
| CAS Number | 13822-56-5 |
| 3D model (JSmol) | `CCN(C)CC[Si](OC)(OC)OC` |
| Beilstein Reference | 1718733 |
| ChEBI | CHEBI:85172 |
| ChEMBL | CHEMBL1626842 |
| ChemSpider | 21559634 |
| DrugBank | DB14035 |
| ECHA InfoCard | 100.109.129 |
| EC Number | 213-817-0 |
| Gmelin Reference | 80536 |
| KEGG | C19205 |
| MeSH | D008411 |
| PubChem CID | 12475 |
| RTECS number | TZ4300000 |
| UNII | F6ST46FVAL |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID0021265 |
| Properties | |
| Chemical formula | C10H25NO3Si |
| Molar mass | 221.38 g/mol |
| Appearance | Colorless to pale yellow transparent liquid |
| Odor | Amine-like |
| Density | 0.950 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | -0.2 |
| Vapor pressure | 0.6 hPa (20 °C) |
| Acidity (pKa) | 10.10 |
| Basicity (pKb) | 6.7 |
| Magnetic susceptibility (χ) | -70.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4200 |
| Viscosity | 1 mPa·s |
| Dipole moment | 3.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 217.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -369.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Harmful if inhaled. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313, P304+P340, P312, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 77 °C |
| Autoignition temperature | 290 °C (554 °F; 563 K) |
| Lethal dose or concentration | LD50 Oral Rat 2413 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat): 2413 mg/kg |
| NIOSH | GVB |
| PEL (Permissible) | Not established |
| REL (Recommended) | No REL established. |
| Related compounds | |
| Related compounds |
3-Aminopropyltrimethoxysilane N-Phenylaminopropyltrimethoxysilane N-Ethylaminopropyltrimethoxysilane N-Methylaminopropyltrimethoxysilane N,N-Diethylaminopropyltrimethoxysilane N,N-Dimethylaminopropyltriethoxysilane |