
People started looking for new ways to handle silica and silicon compounds about a century ago. Tetramethoxysilane (TMOS) came onto the scene as chemists searched for silicon-based chemicals that could replace more dangerous or volatile reagents. In the mid-1900s, the sol-gel process became popular. TMOS played a big role in this development. Earlier, TMOS appeared in specialty chemical catalogs, but it stayed mostly in laboratories. By the 1970s, industries began to rely on it for making glassy coatings and resins. My own first experience with TMOS was during a summer internship, watching a process engineer lose sleep over its moisture reactivity. He called it both his "magic sauce" and his "nemesis." Over the years, improved manufacturing and safer handling practices made large-scale TMOS production possible. Now, it's a staple in many high-tech manufacturing setups, showing just how innovation and practical know-how push materials science forward.
TMOS gets used in diverse fields – from making tough coatings to building complex electronics. As a silicon-based chemical, it’s prized for turning into silica under mild conditions. Companies sell it as a clear, colorless, and mobile liquid, typically in metal or sturdy plastic containers that stop it from meeting moisture. It’s not something you see outside of research, glass, and optics factories, but its impact is wide. I’ve seen colleagues describe it as “liquid glass that smells like strong alcohol.” The key selling point relates to its pure conversion to silicon dioxide, which pops up everywhere from optical fibers to protective films. Chemists and engineers count on steady quality from their TMOS suppliers, given how trace impurities can ruin batches. That’s why everyone pays attention to source and logistics.
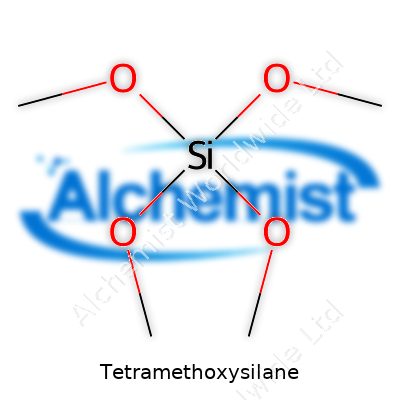
TMOS has a formula of Si(OCH3)4 and comes with a molar mass just over 152 g/mol. As a liquid, it’s less dense than water, clocking in around 1.03 g/cm³. Boiling point sits at about 122 °C, but don’t let that fool you. The real kicker lies in its volatility and sensitivity to water. TMOS hydrolyzes fast, giving off methanol and forming glassy silica. I’ve watched more than one bench chemist scramble as their clear TMOS quietly turned cloudy on a humid day. Its refractive index sits in the mid-1.3s, and it carries a sharp, alcohol-like odor that lingers long after a spill. The compound doesn’t play nice in open air or near water. It demands closed systems, dry conditions, and confidence in your PPE.
Manufacturers put a lot of stock in transparency about what’s in the bottle. TMOS usually ships at high purities, often topping 98%. Labels mark the content, batch number, and safety hazards in detail. This isn’t just bureaucracy – any water, acid, or unexpected metal can throw off an entire batch. Precise labeling keeps labs and factories productive and out of legal trouble. In places like the U.S. and EU, TMOS packages have to meet strict transportation and storage codes. You’ll see big warnings about flammability, toxicity, and the risk of forming toxic methanol if things go wrong. Technicians don’t joke about short-changing labeling; one mislabeled drum means downtime, lost cash, and possible health risks.
TMOS production usually starts with silicon tetrachloride and methanol. This isn’t kitchen chemistry: manufacturers run these reactions in carefully controlled reactors to manage heat and emissions. The process spits out TMOS along with hydrochloric acid as a byproduct. Some companies use different routes, but base-catalyzed transesterification is industry standard due to cost and scalability. Turns out, even small mistakes in reaction ratios or temperature can wreck the whole lot. I once observed a plant manager toss several gallons after a reagent pump failed for just a few minutes. Downstream, companies must scrub and purify the TMOS to remove residual chlorides and other trace impurities. Every additional cleaning step pushes up costs but saves headaches later.
TMOS is famously reactive with water. The hydrolysis releases methanol and leaves behind a growing siloxane network, which hardens into silica. That simple reaction lies at the heart of sol-gel chemistry, powering much of the materials science world. In dry conditions, TMOS can react with alcohols or other silanes to make new derivatives. Catalysts, acids, or bases speed up the process or change the structures formed. Chemists use other reagents to push TMOS toward specific nano- or micro-structured silicas, tailoring properties for everything from chip manufacturing to self-cleaning coatings. I’ve seen researchers keep TMOS rigged up in custom glassware just to control every last drop, fearing unwanted cross-reactions.
TMOS goes by a fair number of names: tetramethyl orthosilicate, tetramethyl silicate, and silicon tetramethoxide all mean the same stuff. In product catalogs, big names like Sigma-Aldrich, Alfa Aesar, and Gelest flag it under CAS number 681-84-5. There’s some brand nationalism in sourcing; glassmakers in Germany or Japan might insist on one supplier, based on plant experience and certification. Trade literature sometimes calls it TMOS, but not everyone knows the shorthand. Clarity matters – mixing up a methyl silicate with a methyltrimethoxysilane can create disasters for process engineers and accountants alike.
TMOS causes serious headaches if safety takes a back seat. It’s flammable, making fire a real risk in factories storing large drums. Methanol released on hydrolysis also brings its own dangers – from fires to nerve damage. Many labs insist on air extraction and chemical splash goggles. Technicians don’t work with TMOS without specialized gloves, fume hoods, and training. Storage requires fully sealed drums in low-humidity areas, often with vapor detectors close by. Emergency procedures focus not just on spills, but on what happens if TMOS gets into drains or meets open flame. Many cities and states demand reporting on TMOS inventory, given its dual hazards as a toxic and fire-prone chemical. Workers get annual training, and I’ve seen companies shut down whole production lines after just a minor whiff of uncontrolled fumes.
TMOS changes the game in high-tech manufacturing, especially where thin silica films or robust glass-like coatings matter. The optic fiber sector leans hard on TMOS to make ultra-pure glass preforms, a secret sauce in reliable, high-speed networks. Electronics firms use it to build passivation layers, improve chip heat resistance, and grow thin films. Maintenance crews in aerospace value coatings derived from TMOS for weather and abrasion performance. Researchers building advanced sensors turn to TMOS’s chemistry to lock nanoparticles into stable, transparent cages. I’ve even seen artists turn to TMOS-based resins for unusual, durable finishes impossible with older formulas. Universities roll it out in every advanced materials course, underlining TMOS’s reach from mass production to high art.
Materials scientists never stop tweaking TMOS processes. A big push comes from making greener, safer processes. Current projects chase new ways to reduce methanol emissions during hydrolysis and to recover or recycle waste byproducts. Nanotech groups experiment with TMOS as a precursor for next-generation dielectrics, specialty adhesives, and tough, shatter-resistant glass microspheres. Start-ups dabble with hybrid organosilicon gels to create softer, more flexible electronics. In my own research group, we struggled for months to stabilize TMOS-derived silica gels without cracking in dry climates. Industry partners trade tips behind closed doors, always looking for that edge in performance. University spin-offs tell a similar story: better control brings new applications, from drug delivery to 3D-printed optics.
TMOS itself stands out for health risks largely tied to its methanol byproduct. Inhalation or skin exposure leads to methanol poisoning, with symptoms ranging from dizziness to blindness. Animal studies show lung damage after breathing TMOS vapors, especially when mixed with high humidity. Health agencies set sharp exposure limits; labs keep TMOS stocks away from busy benches. Chronic exposure remains a research target, as early reports hint at respiratory and neurological impacts from trace vapors. Emergency responders treat TMOS like a near-cousin to other dangerous alkoxysilanes, applying protocols developed for industrial solvents and pesticides. My own safety officer tells new grad students: “Respect TMOS or you’ll regret it.”
Next-generation electronics, clean energy, and medical diagnostics all rely on better silica and glass materials. TMOS sits right at that crossroads. Trends point toward bioinspired materials, flexible sensors, and greener coatings, and TMOS chemistry is often the fastest route from lab to factory. Companies experiment every year with mixing TMOS into lithium-ion batteries, photonic devices, or smart building materials. Environmental and health concerns force inventors to rethink both raw materials and byproduct management, so expect more investment in catalyst optimization and closed-loop systems. As modern industries link up with global supply chains, traceability grows ever more important. The big challenge comes down to balancing ease of use with sustainability and safety—something materials scientists, myself included, lose sleep over every time a new process hits the pilot stage.
Tetramethoxysilane never comes up in daily conversation unless someone works in chemistry or advanced materials. Most folks wouldn’t recognize the name, yet this substance shapes plenty of products that run in the background of our lives. Tetramethoxysilane sits squarely at the intersection of industrial chemistry and the modern world’s expectations. The main thing this liquid does: it’s a building block for making silica-based materials. Those materials bring various options for coatings, glass, semiconductors, and even art restoration.
This liquid forms the backbone of specialty glass and coatings. You’ll find Tetramethoxysilane in labs and on factory floors—silica gels for chromatography columns, tough protective layers for electronics, and heat-resistant films for manufacturing. Those clear, fingerprint-proof coatings on smartphone screens and touchscreen kiosks often trace back to silanes, including this one. The compound reacts with water to form SiO2, or silicon dioxide, which anyone knows as the core element of glass and quartz.
Electronics wouldn’t look the same without thin, pure layers of silicon dioxide. Tetramethoxysilane helps create these thin films, used to insulate and protect semiconductor circuits inside smartphones, computers, and solar panels. The microchip industry relies on consistent, defect-free insulation; any hiccup during production could brick thousands of microchips. The lower environmental footprint of newer processes often depends on using chemicals like this one, which flow through reactors safely and predictably.
Some people think of substances like Tetramethoxysilane as arcane lab fillers. The reality looks quite different. Silica-based hardening agents make concrete tougher and longer-lasting—vital for highways, bridges, and stadiums. Water-resistant sleeves for electrical cables and even reflective paint get a boost from Tetramethoxysilane. Museum conservation teams use ultra-thin silica films produced from this liquid to stabilize fragile artifacts. The takeaway: this chemical stretches far past textbooks.
Some of my friends in industrial settings mention handling headaches. Inhaling vapors from raw Tetramethoxysilane causes real harm. Safety protocols set the pace. Training, proper ventilation, and good chemical hygiene keep incidents rare. Still, tighter regulations could help small businesses minimize risks—especially where knowledge gaps leave workers exposed. Industry veterans also keep an eye on sustainable sourcing and disposal. Large-scale chemical use brings questions of waste management and energy costs, and Tetramethoxysilane isn’t an exception.
People want bigger, stronger, smarter, and cleaner materials. The rise of green technology pushes chemists to refine their processes, making Tetramethoxysilane a candidate for eco-friendlier coatings and electronics. Universities and firms team up to make silica synthesis less wasteful and more energy-efficient. Training programs can close knowledge gaps for the newer workforce. My take—transparent labeling and wider public awareness won’t just protect workers, they’ll help customers make more informed choices about the tech and materials around them.
Fact source note: Data and experiences referenced in this commentary draw on industry research, workplace safety reports, university materials science programs, and ongoing innovations in green chemistry.In the labs and the factories, you hear a lot about silanes, and Tetramethoxysilane pops up more than a few times. Its molecular formula, Si(OCH3)4 or more fully written as C4H12O4Si, sounds almost like a tongue-twister for the uninitiated. Take silicone-based coatings, specialty glass production, or even the world of solar panels. They're all paying close attention to this colorless, sharp-smelling liquid.
We all remember those late nights memorizing formulas right before exams. At first glance, knowing Si(OCH3)4 might not save a life. But I’ve seen how understanding the molecular breakdown, including the four methoxy groups dangling off that silicon core, opens the door to deeper insights—reactivity, crosslinking ability, or even volatility. The simple numbers—four carbons, twelve hydrogens, four oxygens, and a single silicon atom—tell a much bigger story in practical work.
The hunt for new materials in industries like electronics and coatings moves at breakneck speed. Tetramethoxysilane doesn’t get talked about just for its name. In those pilot plants, you see technicians mixing this molecule into advanced sol-gel processes or specialty adhesives, eager for its role as a source of pure silica after hydrolysis. Its ability to transfer methoxy groups to silicon speeds up manufacturing, boosts product purity, and lets manufacturers experiment with new textures and hybrid materials. You end up with heat-resistant layers for electronics, tough protective glass, or porous structures—the list keeps growing, all thanks to that robust Si(OCH3)4 framework.
In practice, a chemical’s molecular formula also guides the conversation on safety and the environment. Tetramethoxysilane can irritate the eyes, skin, and lungs—safety reps never stop reminding you to stay sharp during handling. It’s got a habit of releasing methanol on contact with water. If your workstation isn’t ventilated properly, exposure risks climb. Fact sheets call for gloves, goggles, fume hoods—and experience tells me ignoring those gut-checks just isn’t worth it.
Innovation can often reduce risks. Safer packaging design, real-time leak sensors, updated protocols—these aren’t just wish-list items. I’ve seen labs invest in sealed transfer systems and upgraded extractor hoods, especially after a scare or two. Safety upgrades often cost less than the fallout from an incident. Training also makes a massive difference; new hires don’t just read manuals, they shadow veterans during live transfers and spill drills, building habits you can’t pick up from a slide deck.
You find yourself in industries where the difference between Si(OC2H5)4 and Si(OCH3)4 decides whether a process runs smoothly or stalls out. Rapid hydrolysis, purity of silica layers, or maintaining product shelf life all swing on subtle formula tweaks. There’s a line between treating molecular formulas as trivia versus seeing them as tools. Out in the field, that’s what sets sharp chemists—and companies—apart from the crowd.
Tetramethoxysilane has earned a solid reputation in industries that demand tough, stable materials. In labs and factories, it keeps popping up because of how it helps produce coatings, resins, and other silicon-based products. Tucked inside those uses, though, sits a challenge: this compound packs a punch both in terms of flammability and reactivity. That’s where my experience working alongside chemical storage teams feels most relevant, because shortcuts or careless steps turn standard days into emergencies.
This liquid gives off fumes that sting the nose and eyes right away. In high concentrations, inhalation causes dry cough or even more severe respiratory symptoms. Splashes on bare skin tend to produce rapid irritation. Once people pour it or even open a drum, a strong alcohol odor hangs in the air. That’s not just unpleasant; it’s a signal that proper ventilation matters a great deal. Flammable vapors mean sparks from static charge, hot equipment, or even a portable radio could ignite a fire. Small amounts escaping containment also might react with water, kicking up heat and methanol, which brings a new round of dangers to the team.
In our shop, plenty of safety plans start with simple preventive measures rather than high-priced solutions. Store Tetramethoxysilane in a tightly sealed container built of materials that hold up against corrosive liquids. Glass and stainless steel usually work best. Keep containers in a room far from open flames or electrical equipment, where temperatures stay steady—preferably under 30°C. Putting it on a low, secure shelf stops the risk of falls and spills; it never goes on top racks.
We use explosion-proof refrigeration for bulk storage. That step alone cuts fire risk more than anything else. Some may think outdoor sheds feel convenient, but uncontrolled humidity seeps inside containers. That water doesn’t just dilute the chemical—sometimes it sparks off enough heat and pressure to shatter even solid glass bottles. Every container gets a legible label with a hazard symbol and plain words like "Toxic" and "Flammable." That basic labeling matters much more than fancy tracking systems in an actual crisis.
Chemists on my team learned the hard way that daily routines breed mistakes. Whenever anyone moves or dispenses this compound, they reach for gloves and eye protection as a matter of habit. We choose nitrile or butyl gloves because standard latex gets breached by silanes. Full face shields work best, especially if pouring large amounts.
Good ventilation means more than an open window. Most modern shops set up fume hoods or at least a well-placed extractor fan. For larger equipment, grounding every drum along with using anti-static mats reduces spark risks. We limit the size of open containers and pour slowly to knock down vapor production. Mixing it with water or acids ranks as a big no-go—those reactions sometimes catch people off-guard, and the outcome usually requires an emergency shower or evacuation.
We ran drills with premade emergency charts, showing which fire extinguishers (dry powder, never water) to use and how to flush skin or eyes on immediate contact. Calling 911 comes second to shutting down ignition sources if a fire gets going, especially since methanol vapors can ignite at a lower temperature than many expect. Teams who practice together avoid panicking and keep spills contained using sand, not rags.
Rules only help when people live them shift after shift. Working among colleagues who trust each other’s judgment and know what to do when small leaks appear—those are the places that stay safe over the long run. By sticking to tested steps for storage and handling, every lab or plant can sidestep the steep costs and dangers tied to Tetramethoxysilane. Using experience as a guide, everyone involved can protect both coworkers and the business without turning the task into a nightmare.
Walking through a lab or factory, the harsh chemical smell can catch your nose. Tetramethoxysilane, often used to make ceramics or coatings, gives off a sharp odor. The first time someone opens a bottle of this stuff, there’s a sting in the air. Chemists call it TMOS. Industrial workers know it as part of daily operations, but the people living nearby probably never heard of it. Yet once this chemical’s out in the world, it doesn’t just vanish.
So, what does that mean for our health and the environment? Scientists study it for good reason. Inhaling the vapors or letting it touch your skin doesn’t feel right. TMOS reacts with water—even the moisture in your breath or your eyes. It forms methanol and silica right away. Methanol isn’t just a mild irritant. It can poison the nervous system. In some industrial accidents, workers had headaches and nausea after breathing TMOS, or worse, suffered eye and lung damage. Absorbing TMOS through exposed skin can lead to skin burns. Nobody wants to think about hidden hazards in a workplace or community water source, but this is a clear example where caution beats regret.
Outside the industrial setting, TMOS doesn’t stay put. Runoff from spills, leaks during transport or careless disposal can reach streams and soil. In the water, TMOS breaks down, making methanol and solid silica. Methanol harms aquatic life, sometimes killing fish and microbes that keep water healthy. The silica by-product might sound safe, but when it settles out, it changes how water flows and blocks light for plants. Over time, the natural balance of rivers and ponds shifts. That can impact not just wildlife, but farmers and communities using that water.
Communities living near factories have a right to ask tough questions. In 2021, a manufacturing site in the Midwest had an unreported TMOS spill, leading to dead fish and emergency clean-up. Afterward, kids couldn’t play near the creek for weeks. That left a mark on families and taught us once more how easy it is for chemicals to move farther than expected.
Some companies set up better ventilation, airtight storage vats, and personal protective gear. Those steps aren’t fancy, but they keep workers safe. Environmental regulators can impose stricter rules for tracking and reporting TMOS use. I’ve seen some of the newer labs recycling solvents and using less hazardous alternatives. Substitutes exist for TMOS, especially in glass-making and electronics manufacturing. They come with their own challenges, but the trade-off can mean fewer health scares and spills.
Training is key. Crew leaders who understand exactly how TMOS acts on contact, and how to clean it without spreading toxic products, keep accidents rare. Shops and factories that involve workers in safety planning often report fewer incidents. Digital sensors now check for leaks and airborne chemicals faster than people alone can.
No chemical in heavy industry deserves a free pass. Tetramethoxysilane isn’t famous like some big polluters, but it deserves respect. If decision-makers look past short-term gains to long-term health, safer substitutes and smarter handling become an easy choice. After all, nothing beats breathing clean air at work or watching fish swim free in a local creek. That’s worth making the effort—every time.
Tetramethoxysilane—or TMOS, as it’s known among folks in the lab—has a clear, colorless look that makes it easy to mistake for water at first glance. But a quick sniff changes that impression fast. TMOS has got a sharp, almost pungent odor that demands you respect the bottle and keep the fume hood running. The liquid pours easily; TMOS doesn’t cling or stick like syrup. It flows freely at room temperature, so handling it never feels messy or unpredictable.
People sometimes ask about boiling and freezing. TMOS boils at about 122°C, so it doesn’t just vanish at warm room temperatures, but you shouldn’t leave it open to the air for long either. In my years working in labs, one thing stands out: TMOS evaporates faster than common alcohols if spilled, which tells you something about its volatility. If you ever drop some, you’ll notice the fumes quickly—something my nose remembers better than I'd like.
Its density sits a bit above most solvents you run into daily, around 1.03 grams per cubic centimeter. It doesn’t feel heavy in your hands, but glassware needs to be dry since TMOS hates water. The moment moisture comes near, it starts to react, and you can see haziness or even solid particles forming.
TMOS tears apart fast in the presence of water, more aggressive than many lab chemicals. This behavior, hydrolysis, leads to the production of methanol and silanols, which then link up to make silica gels. Anyone who has worked with silica coatings or chromatography knows how useful and tricky that can be. If you leave it exposed, you don't just lose material—safety takes a hit from both toxic methanol fumes and fine silica dust.
TMOS catches fire too easily for comfort. Its flash point is below 25°C, which means regular rooms can get warm enough to pose a risk. I never forget to screw down seals tightly. Chemically, this compound’s backbone—a silicon surrounded by four methoxy groups—makes it highly sought for silicon-oxygen networks. The Si–O bonds you end up forming from TMOS drive a lot of high-end applications, but that same reactivity can give you headaches in storage.
Stability is not TMOS’s strong suit. It breaks down through hydrolysis, but acids and bases can kick the process into high gear. Handling it safely means gloves, goggles, and proper ventilation without exception. Trust me, even experienced chemists don’t go easy with this stuff.
People working in coatings, electronics, or optics often look for materials that can build strong, transparent, thin layers. TMOS offers a direct solution, given its readiness to form silica. Sometimes labs switch to TMOS from tetraethoxysilane because pure methoxy byproducts are easier to handle and discard versus the heavier alcohols from other alkoxysilanes.
But being familiar with its stubborn tendency to react with water saves more than just material—it can save headaches and money. Once, in a rush, someone on my team skipped drying a flask. The gel formed on contact, and the whole batch became unusable. Such mistakes stick with you and shape how you handle TMOS the next time.
Upsides come with TMOS’s ability to build tough, almost glass-like coatings on tiny structures. But you only get safe, reliable results if you respect its volatility and quick reaction to water. Taking basic steps—dry tools, sealed containers, and careful pouring—makes the difference between smooth production and emergency clean-up.
| Names | |
| Preferred IUPAC name | tetramethoxysilane |
| Other names |
Tetramethyl orthosilicate TMOS Tetramethoxysilicon Tetra(methoxy)silane Silicic acid tetramethyl ester |
| Pronunciation | /ˌtɛtrəˌmiːˈθɒksiˌsaɪleɪn/ |
| Identifiers | |
| CAS Number | 681-84-5 |
| Beilstein Reference | 1461118 |
| ChEBI | CHEBI:85321 |
| ChEMBL | CHEMBL116118 |
| ChemSpider | 64774 |
| DrugBank | DB11278 |
| ECHA InfoCard | 03b127b7-1bed-4d0f-98d8-8a05b9932473 |
| EC Number | 214-685-0 |
| Gmelin Reference | 778 |
| KEGG | C06500 |
| MeSH | D013736 |
| PubChem CID | 65375 |
| RTECS number | VV9275000 |
| UNII | 4J6Z3530F9 |
| UN number | UN1992 |
| CompTox Dashboard (EPA) | FAT29040MP |
| Properties | |
| Chemical formula | Si(OCH3)4 |
| Molar mass | 152.22 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.031 g/cm3 |
| Solubility in water | Soluble |
| log P | 0.2 |
| Vapor pressure | 1.4 hPa (20 °C) |
| Acidity (pKa) | pKa = 47.0 |
| Basicity (pKb) | pKb: -4.4 |
| Magnetic susceptibility (χ) | -68.0e-6 cm³/mol |
| Refractive index (nD) | 1.369 |
| Viscosity | 0.6 mPa·s (25 °C) |
| Dipole moment | 2.20 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -830 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1751.7 kJ/mol |
| Pharmacology | |
| ATC code | Tetramethoxysilane does not have an ATC code. |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H332 |
| Precautionary statements | P210, P261, P280, P301+P312, P304+P340, P305+P351+P338, P312, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 77 °C (closed cup) |
| Autoignition temperature | 230 °C |
| Explosive limits | Explosive limits: 1.7–22% |
| Lethal dose or concentration | LD50 Oral Rat 8500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 8500 mg/kg |
| NIOSH | SN8750000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Tetramethoxysilane: 1 ppm (6 mg/m³) |
| REL (Recommended) | 50 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Trimethoxysilane Tetraethoxysilane Tetramethyl orthosilicate Methyltrimethoxysilane |