
Plenty of companies in the chemical industry have searched for ways to make high-performance silane coupling agents. The journey leading up to triacetoxyethylsilane started with the basic family of trialkoxysilanes in the mid-1900s. Researchers wanted new agents to help finish surfaces and bring more value to industrial adhesives. Out of many experiments, scientists discovered that introducing acetoxy groups into ethylsilane molecules added efficiency in hydrolysis and better reactivity in mild conditions. Over time, this compound transitioned from a laboratory curiosity to a popular ingredient in specialty coatings, sealants, and silicone elastomers, mainly because of its versatility and improved performance over older silanes.
Triacetoxyethylsilane stands out because it plays a double role both as a binding promoter and as a cross-linking agent. In industrial settings, it makes its mark by improving the stickiness and weather resistance of sealants, adhesives, and even protective coatings. Many suppliers carry other names for the product, such as Ethyltriacetoxysilane or Silane, triacetoxyethyl-. For the consumer or the researcher, the labeling will often highlight its main identifiers — typically including a company-specific product code, purity percentage (usually upward of 98%), and sometimes a reference to its physical form, clear and slightly oily liquid.
In the lab, triacetoxyethylsilane appears as a colorless, transparent liquid with a sharp vinegar-like scent due to the acetoxy functional groups. It measures a boiling point in the range of 114–118°C at reduced pressure, and its density sits near 1.05 g/cm³ at 25°C. As a non-polar compound with good solubility in organic solvents but strong reactivity with water, precautions during storage become critical. The substance reacts briskly with water, producing acetic acid and silanols, which can undergo further cross-linking or polymerization. Careful handling, tightly sealed containers, and cool dry storage environments give the best results for maintaining its shelf life.
Technical datasheets for triacetoxyethylsilane reflect the need for detail. Labels highlight batch numbers, the net mass, purity levels, and recommended storage conditions. Bulk storage, even outside a highly regulated setting, stays away from open air, preventing the release of corrosive vapors. Some producers stamp hazard labels related to its skin and eye irritation risks and flammability. Safety data sheets bring attention to the need for splash goggles, chemical-resistant gloves, and positive venting during use. Downstream labeling emphasizes possible mixtures with silicon-based oils, showing clear QA boundaries.
Manufacturing triacetoxyethylsilane usually involves reacting ethyltrichlorosilane with acetic acid in the presence of a base or, at times, a metal catalyst, to substitute the chlorine atoms with acetoxy groups. The resulting compound, after purification, gives a clean, colorless product by distilling off excess acetic acid and side products. The procedure stands as efficient and scalable, favored in both bulk and specialty settings. Most commercial batches run in closed systems with strict monitoring for temperature, pressure, and byproduct removal to avoid loss of yield.
The reactivity of triacetoxyethylsilane gives it significance in modern material science. On exposure to water, it hydrolyzes rapidly, releasing acetic acid and forming reactive silanol groups. These silanols interact with a wide array of substrates, including glass, metal oxides, and ceramics. Its crosslinking function links polymeric chains in silicone rubbers, boosting tear resistance and resilience. For modified grades, small changes in the ethyl side chain produce derivatives suited to various industrial needs, broadening application in new polymers and composites.
Across suppliers and purchasers, triacetoxyethylsilane wears a few different hats. Some catalogs list it as Ethyltriacetoxysilane; others mention Triacetoxy(ethyl)silane, or its chemical shorthand, C8H16O6Si. Well-established companies put their own spin on the naming: “Silquest A-162” or “Dynasylan 4102” serve as recognizable industrial marks, helping end-users quickly spot the right material for specific applications. Locating consistent synonyms guards against confusion—critical in research, procurement, and regulatory registration.
This silane brings more than convenience to a formulation; it demands respect for safety. Its contact with water, even ambient moisture, generates acetic acid vapor, which can irritate skin, eyes, and mucous membranes. Technicians report stinging sensations and even burns after accidental exposure. Storage away from humid areas and good local exhaust ventilation reduce risk. In larger operations, standard practice dictates pre-use training, emergency eyewash access, and EHS oversight. Incidents of respiratory distress highlight the danger of ignoring fume management or improper PPE—routine hazards in the specialty chemical industry.
Industry professionals use triacetoxyethylsilane most in building and construction products—especially those seeking tough, weather-resistant sealants and adhesives. Its value shows up wherever a long-lived silicone bond can make or break a product, like façade coatings, bathroom caulks, and kitchen sealants. Researchers and quality managers have pointed out improvements in cure speeds and durability for glass insulation units treated with this agent. Labs working with automotive and aerospace elastomers choose this compound for precision mold release and surface treatment. Its presence makes the difference in electronics encapsulation and even in coatings on photovoltaic modules.
Active research teams continue to explore what happens when triacetoxyethylsilane meets new partners—like high-surface-area fillers or engineered nanoparticles. Studies have measured its efficacy in producing more robust hydrophobic surfaces, corrosion-resistant layers, and hybrid organic-inorganic films. Ongoing patents and academic work keep pushing boundaries, including its pairing with renewable adhesives and foams for greener construction methods. Technical experts gather at conferences to compare accelerated aging results and to brainstorm on breakthrough surface chemistries. Its adaptability and reactivity promise a longer, more diverse future as industries ask for smarter, tougher, and more sustainable systems.
Toxicologists have scrutinized triacetoxyethylsilane, dissecting its breakdown products—mainly acetic acid and ethylsilanol. Short-term exposure in lab animals gave eye and respiratory irritation, mostly due to acetic acid production on hydrolysis. There have been in-depth studies measuring occupational exposure limits, verifying that with good controls, exposure remains manageable. Environmental scientists focus on its rapid breakdown in moist conditions; its hydrolysis results in relatively benign silicon dioxide, but persistent emissions of acetic acid in closed indoor environments raise questions for air quality management. Researchers and regulators keep improving guidelines to make both workspaces and communities safer.
The next phase for triacetoxyethylsilane seems set for broader use in energy-efficient construction, long-lasting infrastructure, and more ecological adhesives. Emerging green chemistry techniques seek to lower manufacturing energy use, cut emissions, and recover all side products. Entrepreneurs and established multinationals want to leverage its chemical flexibility—the expansion into modified cross-linkers means it will probably feature in future 3D-printed building materials and even smarter, self-healing polymers. As regulations toughen around volatile organic compounds and workplace safety, ongoing research continues to refine uses, synthesize variants with reduced hazard, and widen the range of sustainable, valuable uses for this distinct silane compound.
Triacetoxyethylsilane often flies under the radar unless you’ve spent real time in laboratories or on production floors. This chemical gets a lot of attention in advanced manufacturing and materials science. In most places where people want to improve the surface properties of a product, this compound finds its way in. My experience working alongside R&D teams taught me that silanes like this one act as trouble-shooters, tackling weak points in materials or molecular structures.
Manufacturers reach for triacetoxyethylsilane when they want to help things stick together or modify a surface to accept coatings or inks. Take glass production as an example. Glass naturally resists painting. Silane treatments let companies print or tint glass strongly and with lasting results. Without this chemical, coatings tend to peel and fade, especially in harsh conditions. In the world of electronics, circuit boards get extra protection thanks to a silane-based primer layer, which keeps moisture out.
A good friend of mine in the sealant industry used to joke that if you see a modern window or a weatherproof joint that lasts, there’s probably a silane working hard in the background. Triacetoxyethylsilane helps silicone-based sealants cure and bind to surfaces. This chemical turns up in construction as an ingredient that makes caulks resistant to mold, water, and sunlight. One testing manager told me that after switching to silane-based compounds, his company’s products handled stress tests much better.
Not every chemist wakes up excited about crosslinking, but for anyone making resins or specialty polymers, silanes become essential. Triacetoxyethylsilane links different molecular chains, making plastics tougher or more flexible. The result is a wider range of uses for manufacturers, from cables to medical devices. A 2022 research summary in the Journal of Applied Polymer Science showed that silane crosslinkers can extend the lifespan of plastics by more than 30%. This means less waste and sturdier end products.
Every time something improves, new headaches pop up. Triacetoxyethylsilane reacts with moisture, which releases acetic acid—a sharp-smelling byproduct that can corrode equipment. Workers have to follow strict ventilation rules and use personal protective equipment. I remember walking into a production line meeting and hearing complaints about gloves wearing out faster than usual. Companies that care about occupational safety regularly review their protocols and swap out gear as needed.
Silane chemistry, just like any industrial process, faces scrutiny for its environmental footprint. Producers who invest in closed-loop systems and better waste management help cut down on chemical runoff and emissions. I’ve seen some teams develop alternative curing methods that use less energy and generate fewer byproducts.
Markets keep demanding higher-performing products, but cost controls and health concerns always nudge companies toward innovation. Research centers are testing bio-based silanes and less reactive alternatives. From my own visits to advanced materials conferences, I see a clear trend: every part of the supply chain, from suppliers to end users, pushes for safer formulations without sacrificing quality. So, triacetoxyethylsilane might not be headline material, but its role shows how the right chemistry changes daily life in ways most of us never notice.
Triacetoxyethylsilane isn’t just a mouthful; its chemical formula, C10H20O6Si, packs in quite a bit of information. It comes from putting together a silicon atom, an ethyl group, and not one or two, but three acetoxy groups. Each piece has its own job in the way this compound behaves. See, chemistry likes shortcuts. Looking at “Triacetoxyethylsilane” straight away, you get the sense of three acetoxy (CH3COO–) groups tagged to a silicon atom, which also holds onto an ethyl chain. So that’s where the formula comes from – count the carbon, hydrogen, and oxygen in each little group and piece them around Si.
I’ve seen how Triacetoxyethylsilane pops up in the lab as a specialized reagent. Not every chemical finds its way into adhesives, polymer science, and surface-modifying treatments, but this one is regularly drawn on for those tweaks. That’s because the silicon atom can play matchmaker between organic groups—those built around carbon chains—and inorganic stuff, like metal oxides on glass or ceramics. This sort of cross-linking isn’t just clever—sometimes it’s the difference between a coating that flakes off in weeks and one that holds strong for years.
It’s easy to overlook how chemistry touches everyday things. The stuff that holds electronic devices together, the coatings inside building panels, these often include silicon-based compounds you’ll never read on a label. Triacetoxyethylsilane is one of those behind-the-scenes players. Acetoxy groups release acetic acid when they react with moisture. It smells like vinegar, sure, but that reaction means the molecule hooks itself tightly to surfaces, especially to materials like glass. It’s not the kind of chemistry that gets flashy headlines, but without it, a lot of modern manufacturing would run into trouble.
Anyone who’s ever opened a bottle of silane reagents will tell you: you want the fume hood running. Triacetoxyethylsilane can irritate eyes, skin, and lungs, and it produces acetic acid vapors as it reacts with water. Gloves and goggles aren’t optional—they’re a must. In the early days of working in a chemical lab, I learned this lesson the hard way after catching a whiff of that strong vinegar smell. The chemical itself might sound exotic, but the safety basics come down to solid PPE and good ventilation.
Plenty of folks in industry see the benefits of silanes for stronger adhesives or better coatings, but the safety side really shouldn’t go ignored. Investing in training for workers and setting up smart ventilation can avoid a lot of headaches—not just literally. It’s also worth double-checking storage guidance. These chemicals again react with water, so humidity control matters just as much as keeping the bottles tightly capped. Factoring in the environmental impact, some companies look to recover or neutralize waste acetic acid too, which helps cut down on emissions.
Triacetoxyethylsilane’s formula—C10H20O6Si—carries weight well beyond textbooks. Think reliable, cross-linking performance, with attention needed for safety both during storage and use. It’s one of the reasons certain materials last, bond, and resist wear in tough conditions. With smart handling, the real-world impact can be both powerful and responsible.
A bottle labeled Triacetoxyethylsilane probably looks like any other chemical container on the shelf, but anyone who’s dealt with organosilanes knows that these are not the sort of compounds you want to treat casually. They can react with moisture in the air and produce acetic acid, so it’s not just about saving money—it’s about staying safe and protecting your environment from unnecessary hazards.
I remember early in my career, a bottle of this stuff was left out after someone finished their prep work. By the next afternoon, the label was already peeling from splashes of acetic acid that had slowly seeped out, and the distinct sharp smell hung in the air. It’s the kind of oversight you only make once. A moment of carelessness had turned a benign shelf into a cleanup job. After that, no one left these reagents out, and every new student got a quick lesson in what not to do.
Let’s cut to the chase: Triacetoxyethylsilane needs a dry, cool spot, out of direct sunlight. Exposing it to open air invites trouble, as it pulls water from the atmosphere and releases acetic acid. So, anyone storing it ought to use tightly sealed, original containers—preferably glass with Teflon-lined caps or suitable chemical-resistant material. No shortcuts with half-screwed lids from shared jars. Each time the bottle gets opened, there’s another risk of exposure, damage, or waste.
I’ve seen some folks store incompatible chemicals next to each other because it saves them a walk across the room. It’s not worth it. Triacetoxyethylsilane should stay clear of water, acids, alcohols, and any area where spills or fumes could mix with the wrong substance. Ventilated cabinets designed for flammables or reactive materials beat the standard closet every time. Don’t get lazy about secondary containment, either—a simple plastic tray catches drips before they become problems.
Sometimes, hand-written tape labels get smudged or misplaced in a busy space. That just leads to more confusion. Invest in printed, chemical-resistant labels that list the full name and hazards. Even in small research teams, a digital logbook helps track who last used the bottle and when it was opened. That minimizes the chance of forgotten, deteriorating containers turning up years later—an all-too-common cause of lab evacuations and equipment damage.
Personal protective equipment deserves a mention. Gloves and eye protection are musts. If you’re opening a fresh container or transferring aliquots, do it in a fume hood. The acetic acid vapors might not seem like much at first, but after an hour in a cramped space, your eyes, skin, and sinuses will disagree. Waste containers should be nearby, clearly labeled for silane residues, not dumped into regular solvents or aqueous waste streams.
Many workplaces still treat proper chemical storage as a chore, rather than the first step in safety. Bringing in regular training, proper signage, and routine audits helps foster a culture where mistakes aren’t just less likely—they’re talked about openly so everyone learns. Using digital inventory systems, smart cabinets with sensors, and regular label checks saves time and, most importantly, stops incidents before they begin.
Triacetoxyethylsilane pops up in specialty chemical discussions, especially among folks in research labs or companies working with advanced coatings and adhesives. Its applications sound niche, but the health and safety of anyone who comes into contact with it are anything but trivial. I learned the hard way in a past job not to brush off chemicals just because they don’t send up an obvious red flag.
A closer inspection of Triacetoxyethylsilane’s safety data reveals something worth every worker’s attention. This compound releases acetic acid on contact with moisture—including the air or your skin. Acetic acid, the same stuff that gives vinegar its acidity, eats away at tissues at high concentrations. If you don’t gear up right, you end up with painful skin or eye irritation. Even mild exposure leaves you with a sting that lingers. Just ask anyone who’s handled leaky containers barehanded.
The inhalation risks shouldn’t be swept aside. Inhaling dust, mist or even the fumes irritates the airways, and for sensitive lungs or asthmatics, that’s courting trouble. Prolonged exposure ramps up the dangers. The nerves and kidneys don’t respond well to chronic, low-level chemical exposure. Somewhere between quick contact and daily handling, there’s a line where risk flips from minor to meaningful. Many workplaces struggle to identify where that line sits, especially with compounds as specialty as this one.
Training beats luck every time I see people handling chemicals. Procedures around Triacetoxyethylsilane need steady reminders: splash goggles on, gloves checked for damage, chemical aprons fastened. Respirators belong on your face, not around your neck. Chemical handling isn’t something you want done by memory or habit, since one careless move lands you in the lab wash station or worse. I’ve seen accidents unfold from shortcuts and used them as examples for better practices. Nobody enjoys a chemical burn.
The U.S. Occupational Safety and Health Administration (OSHA) and Europe’s REACH system factor in chemicals like Triacetoxyethylsilane, flagging them for handling controls. Employers hold the responsibility for proper labeling, accessible safety data sheets, and regular air quality checks. Skipping those steps piles up liability and puts lives at risk. Labs and plants moving from outdated chemicals to modern alternatives should audit their storage each year. It’s surprising how often people keep forgotten bottles on cluttered shelves.
Basic engineering measures tighten up the margin for error. Fume hoods and sealed containers reduce spills and fumes. Clean work areas and immediate wipe-down regimens make a noticeable difference in the accident rate. People learn not just from procedures but from seeing senior staff take precautions seriously. Safety culture grows when every person, from supervisor to new hire, treats protective gear as part of the uniform.
Triacetoxyethylsilane isn’t the scariest chemical around, but it carries enough risk to deserve real respect. Proper handling, working fume hoods, good training—all these cut down not only on health incidents but also on the small, daily mishaps that slow everyone down and eat into budgets. In the end, it’s about respecting your own health and setting an example for the next person who’s handed the bottle.
Triacetoxyethylsilane isn’t a chemical most people keep on a shelf at home. In labs and workshops, though, it helps with all kinds of silicon-based projects. Still, skipping safety basics with this stuff lands folks in trouble fast. Vapors sting the eyes and nose. Spills eat through gloves if you pick the wrong kind. The liquid irritates skin. Left lying open on a bench, it can even sprinkle your workspace with flammable fumes.
I’ve worked in a few research labs through the years, watching colleagues rush for deadlines. Silanes get treated like any other bottle—until something splashes outside the fume hood. One time, a researcher wore those thin clinic gloves, sure they’d hold up. Didn’t notice the burn on her hand until it started to itch and turn red. The cleanup took twice as long because she hadn’t grabbed the right spill kit.
Nobody enjoys suiting up with goggles, face shields, or heavy gloves. But these basics change the story. Splash-proof goggles stop fumes from sneaking into your eyes. A face shield cuts the risk from spray or drops. Nitrile or butyl rubber gloves put up a decent fight against silanes, while latex gloves don’t last a minute. I always tell new folks, set up every experiment inside a ventilated hood. This habit keeps the air safer for you, not just the next worker.
Fume hoods make a difference. They clear out vapors before you breathe them in. I remember working in an old lab with weak ventilation. Bottles of silane got opened anyway, and a strange vinegar odor would linger for hours. No headaches, no quick trips to the emergency room—but a day full of warning signs for anyone paying attention.
Triacetoxyethylsilane breaks down with water, sometimes in explosive fashion. A damp shelf, a loose lid, and you’re set up for a mess. All it takes is a forgotten bottle, a leaky roof, or a cleaning spill. Folks learn quickly to seal bottles tightly and to keep them away from sinks or open windows. I’ve seen smart storage mean the difference between a scary incident and a routine check on chemicals.
Spilled silane shouldn’t go into the sink. Soak up drops with inert absorbents, then toss them into chemical waste. A mop or rag just spreads contamination. Everyone in the lab ought to know where to find spill kits—right along with emergency eyewash stations. These small actions turn a bad day into a memory instead of an accident report.
Sitting through safety training sometimes feels like busywork. But real stories—yours or a coworker’s—stick with new hires better than any bland poster on the wall. Once you’ve learned from the scrapes and close calls, you pass on tips people trust. Stick close to those who treat every chemical as a risk to respect. They’re the ones who head home healthy at the end of every shift.
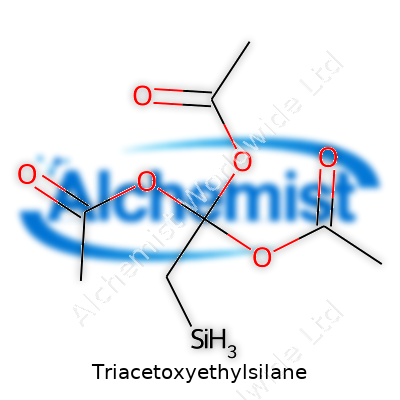
| Names | |
| Preferred IUPAC name | Triacetoxy(ethyl)silane |
| Other names |
Ethyltriacetoxysilane Triacetoxy(ethyl)silane |
| Pronunciation | /traɪ.əˌsiː.tɒk.siˌiː.θɪlˈsɪ.leɪn/ |
| Identifiers | |
| CAS Number | 17689-77-9 |
| Beilstein Reference | 3568736 |
| ChEBI | CHEBI:132747 |
| ChEMBL | CHEMBL4685047 |
| ChemSpider | 123499 |
| DrugBank | DB22071 |
| ECHA InfoCard | 17d7c714-91e7-4c1d-8199-87a0db430d8c |
| EC Number | 263-041-4 |
| Gmelin Reference | 85458 |
| KEGG | C18607 |
| MeSH | Triacetoxyethylsilane |
| PubChem CID | 6851100 |
| RTECS number | VV7780000 |
| UNII | MX4922J0TY |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C8H14O6Si |
| Molar mass | 320.39 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.993 g/mL |
| Solubility in water | insoluble |
| log P | 0.5 |
| Acidity (pKa) | 6.2 |
| Basicity (pKb) | -5.2 |
| Magnetic susceptibility (χ) | -73.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.397 |
| Viscosity | 5 mPa·s (25 °C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 532.8 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | There is no ATC code assigned to Triacetoxyethylsilane. |
| Hazards | |
| GHS labelling | GHS02, GHS05 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | Precautionary statements: "P261, P280, P304+P340, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-2-W |
| Flash point | 83 °C |
| Lethal dose or concentration | LD50 Oral Rat 2307 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 2300 mg/kg |
| NIOSH | NYP930000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 200 mg/m3 |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Triethoxysilane Triacetoxymethylsilane Trimethoxyethylsilane Tetraacetoxysilane |