
The path to vinyltriethoxysilane points back to the rapid advances in organosilicon chemistry of the twentieth century. Innovators in the 1940s began tinkering with silicon compounds in the race to bridge the gap between inorganic and organic materials. Researchers got serious about hybridizing vinyl groups with silicon, aiming for stronger and more versatile polymers. Efforts paid off through industrial syntheses that enabled large-scale production. These discoveries emerged during the postwar boom, when new materials meant progress for construction, transportation, and electronics. This compound’s journey tells the story of how chemical curiosity eventually shaped entire industries, answering the call for reliability in everything from glass composites to cable jacketing.
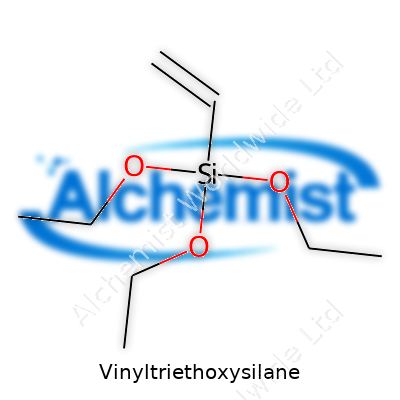
Vinyltriethoxysilane stands out as a potent silane coupling agent. Its molecular formula, C8H18O3Si, embodies a silicon core flanked by a vinyl group and three ethoxy wings. Used as a clear, colorless to light yellow liquid, this material needs no fancy description or hidden use; it’s about linking the physical worlds of polymers and mineral surfaces. The real magic of this molecule comes from its ability to graft organic and inorganic phases, opening up unique possibilities for durability and performance, particularly with fillers in plastics, rubbers, and surface treatments.
This silane sports a boiling point near 161°C under standard pressure, with a density hovering around 0.9 g/cm³ at room temperature. It flashes off at about 41°C—attention to fire risk cannot be overstated. As for solubility, vinyltriethoxysilane dissolves in organic solvents but reacts in water, where the ethoxy groups swap out for silanols through hydrolysis. It has a pungent odor, and traces of moisture will kickstart its continuous transformation. With a refractive index of about 1.395 and vapor pressure that rises with temperature, its volatility demands respect during handling and storage.
Technical grades of vinyltriethoxysilane typically achieve purities greater than 97%. Labels give specifics: Molecular weight hovers at 190.3 g/mol, and lot numbers stay visible for traceability’s sake. Storage recommendations read like a checklist for safety: airtight drums, cool dry spaces, and careful separation from acids or bases. Compliance with transportation and international safety codes (such as UN 1993 for flammable liquids) must figure into any shipping or warehouse plan. Export and import regulations, GHS labeling, and up-to-date MSDS sheets round off the regulatory landscape. Only trained personnel handle this compound in regulated facilities, wearing proper PPE such as chemical splash goggles and gloves made from nitrile or neoprene.
Synthesizing vinyltriethoxysilane generally involves a hydrosilylation reaction, where triethoxysilane reacts with vinyl chloride or an equivalent vinyl source under platinum or rhodium catalysis. Precision controls the yield and purity. Chemists carefully exclude moisture, seal reactors with inert atmospheres, and push catalysts to work at moderate temperatures, limiting side reactions. Some producers favor continuous over batch processing for throughput and consistency. After reaction, distillation purifies the product, stripping out byproducts and traces of catalyst. What leaves the reactor is a high-purity liquid ready for functionalization or direct application.
The essence of this silane’s value lies in its vinyl and ethoxy groups. Hydrolysis kicks in and turns the ethoxy groups into silanols, especially when exposed to moisture. Once silanols form, they rapidly condense onto mineral surfaces such as glass, silica, or metal oxides. Chemists exploit this reactivity to anchor polymers securely to inorganics. The vinyl group, on the other hand, participates in polymerization, crosslinking with unsaturated resins like polyethylene, EVA, or polyvinyl chloride. Modifications can involve copolymerization, allowing fine-tuning of flexibility and strength in end materials. The molecule works well in sol-gel processes, and custom silane blends often emerge for optimized adhesion or weatherability in finished goods.
This compound shows up in literature and commerce under several aliases, including triethoxy(vinyl)silane and ethenyltriethoxysilane. Trade names differ with suppliers—A-151, Silquest VS, GENIOSIL GF 56, and Dynasylan® VTEO are just a few. Manufacturers and researchers stick to the same chemical backbone but always list CAS number 78-08-0 for clarity. Such variety in naming sometimes creates confusion, but the molecular signatures all echo the same reactive possibilities.
Handling vinyltriethoxysilane never means cutting corners. The highly flammable nature leads to strict access control and grounding in storage settings. Vapors irritate eyes and respiratory tracts. Industrial users rely on forced ventilation and explosion-proof equipment. Staff receive hands-on training focused on spill cleanup—absorbents, containment, and prompt disposal reduce fire and environmental risk. Emergency proceedings align with OSHA, REACH, and other local frameworks. Any waste gets designated as hazardous, headed for specialized incineration or secure landfilling. Regular workplace air monitoring helps spot any leaks before risks grow.
Vinyltriethoxysilane empowers a wide swath of industries. Its major home sits with cable insulation and sheathing for modern telecommunications, where improved adhesion bolsters long-service life and resistance to environmental stress. In construction, it functions as a primer or additive for glass fiber composites, gypsum boards, and sealants—keeping structures tight and durable. Paints, adhesives, and coatings depend on it for binding fillers and pigments, ensuring lasting bonds even in wet or aggressive chemical environments. Automotive manufacturing turns to this silane to enhance scratch resistance and weatherproofing. The electronics industry, concerned about signal loss and moisture ingress, integrates the compound in circuit board laminate manufacture.
Researchers have thrown significant effort into pushing vinyltriethoxysilane applications further. Surface chemistry advances focus on lowering the environmental footprint and maximizing recyclability of silane-treated materials. Laboratories explore dual-function silanes, blending other functional groups for new performance benchmarks. In nanotechnology, this compound features in fabricating hybrid materials, with controlled interfaces translating to stronger, lighter products. Academic studies measure how well silanized surfaces resist corrosion or biofouling—real advantages in marine or medical gear. R&D teams respond to supply chain shifts and regulatory updates by searching for more sustainable catalysts, lower-emission production, and safer packaging.
The safety profile of vinyltriethoxysilane pulls insights from years of industry and lab scrutiny. Short-term exposure can cause acute irritation of skin, eyes, and mucous membranes. Studies in rodents show low acute toxicity, with high doses needed to cause lethal effects. Chronic exposure raises questions, as repeat inhalation could inflame airways or sensitize personnel to chemical allergies. Some breakdown products, including ethanol formed during hydrolysis, pose their own hazards if released indoors. Safety data sheets draw from OECD test guidelines and regular toxicological monitoring. Regulators set strict occupational exposure limits. Environmental research tracks its fate after waste or accidental release—the compound hydrolyzes and degrades, but pains remain to avoid uncontrolled discharges and to treat effluent water.
The potential for vinyltriethoxysilane won’t slow down as global demand grows for lightweight composites, advanced electronics, and climate-resilient buildings. Sustainable chemistry movements push the industry toward greener synthesis routes and bio-based feedstocks. Digitization and automation promise tighter process control, yielding less waste and finer-tailored grades. As recycling streams for plastics and composites ramp up, engineers look for silanes that can help delaminate or refunctionalize surfaces. Active collaboration between chemical makers, universities, and government labs seeds the next generation of innovation—reshaping what silanes can accomplish as building blocks of twenty-first-century technology. The drive for safety, health, and minimal environmental impact threads through it all, aiming for a future where strong, smart materials benefit society without undue cost.
Vinyltriethoxysilane shows up in the real world more than people realize. You won’t likely see it sitting on a shelf, but it works its magic behind the scenes in everything from power cables to everyday building materials. This chemical plays a big role in getting plastics to grab onto glass, metal, or even minerals inside composites. You get scrappy materials that stick together and last longer.
Take a look inside a cross-linked polyethylene (XLPE) cable. The silane treatment makes the cable tougher, stops moisture from creeping through, and keeps electrical properties stable. Factories feed in vinyltriethoxysilane during wire and cable production, and it actually links the polymer chains together. This connection means insulation lasts for years—crucial for buried or underwater lines. Not bad for a clear liquid.
Go into the world of plastics, and you see vinyltriethoxysilane helping out as a coupling agent. Filler particles like silica tend to resist mixing with plastics, which causes trouble for engineers. Silane jumps in, tying those particles to the plastic so the finished part turns out stronger and more reliable. Tires, window seals, gaskets, and kitchen gadgets all rely on this step.
Manufacturers in construction use silane to treat glass and cement. This creates surfaces that reject water and work better with paints or coatings. Skyscraper windows hold up to storms. Floor tiles avoid breakdown from chemicals and moisture. Outdoor structures take a beating and last longer thanks to silane treatments.
It all boils down to getting the right materials to last in all sorts of environments. Silane technology supports big improvements over old-school adhesives, which often broke down under stress or in weather extremes. Now, a bridge deck or cable insulation doesn’t just survive—it pushes the limits set by bare rubber, glass, or plain plastic.
For anyone working to build smarter, more reliable products, understanding the chemistry pays off. Vinyltriethoxysilane also helps lower the need for harsh chemicals in paints and plastics. With better adhesion, manufacturers use less toxic additives. It’s not a cure-all, but, by creating strong chemical bonds, the need for extra glue, solvents, or finishing steps drops.
With more chemical use comes bigger health and safety questions. Many silanes, including vinyltriethoxysilane, can cause skin and eye irritation if workers skip safety steps. Factories need ventilation and proper training. The waste or runoff from these plants could create problems for local water sources if not managed closely. Transparency in how and where these chemicals end up will matter more as rules get tighter.
Cleaner production methods and better recycling for silane-treated products could tackle some risks. Research keeps moving ahead, and new generations of silanes might drop the health hazards while delivering the same punch. Companies that build with silane should invest in worker safety and waste management. Public pressure on chemical handling means no shortcuts—companies showing responsibility will earn trust, avoid fines, and push the industry ahead. Vinyltriethoxysilane proves how chemistry gives us new tools, but also demands respect. If we handle it right, everybody can benefit from what this molecule offers.
Vinyltriethoxysilane carries the formula C8H18O3Si. Three ethoxy groups attach to a silicon atom, leaving a vinyl group as a fourth bond. That set-up sounds technical, but in practice, this little molecule supports a lot of work behind the scenes in construction, automotive parts, adhesives, coatings, and even electronics.
Most folks don’t glance at their laminate flooring and wonder if a silane coupling agent made it possible, but the effects show up all the time. In construction, adhesion doesn’t always come easily. Fillers and reinforcement materials like glass fiber struggle to bond with organic resins in plastics or composites. Vinyltriethoxysilane steps up as a chemical hand-shake. Its vinyl group bonds well with organic resins—think polyester or epoxy. The trimethoxysilyl portion likes glass, metal, or mineral surfaces. This dual personality lets it build bridges between two pretty stubborn groups of molecules.
I’ve done some repairs where silicone sealants don’t sit right on a dusty, crumbling surface. Trying a product that lists a silane additive changes how firmly the joint sets, especially in high-moisture settings. Builders and repair techs who spend hours fixing surfaces notice how little chemical tweaks help roofs, tiles, or window seals survive changing weather and humidity.
With all benefits, health and safety come into play. Vinyltriethoxysilane doesn’t sound friendly—it doesn’t taste or smell pleasant either. Companies making coatings and adhesives face tighter rules about exposure and emissions. Long-term inhalation or skin contact can set off irritation, so using proper ventilation and gloves in the shop or factory becomes a must. Tools and workspaces should get cleaned after use, since silane residues can react with water in the air and form slippery films.
Research from the U.S. National Library of Medicine and material safety datasheets back these points: safe handling relies on education, personal protection, and smart storage. Chemical manufacturers already run tests on emissions and product breakdown, but keeping up with new findings helps workers avoid unknown risks. It pays to read up before using any chemical—even seemingly routine ones like silanes.
Workers on the ground don’t always get a say in the ingredients packed into bulk chemicals or sealants. Still, calls for transparency keep growing. Labels explaining exact content, possible hazards, and environmental impact can make a difference. Consumer pressure sometimes gets lost in technical supply chains, but standards committees now require greater reporting. Shoppers and contractors should ask for those details. Over time, the demand for safer additives influences what gets produced and stocked on store shelves.
Marginal gains from improvements in chemical bonding save lots of waste and failed repairs. Choosing to use tested, documented silanes with up-to-date safety checks supports a longer product life and healthier working conditions. Vinyltriethoxysilane plays a quiet but powerful role—one well worth understanding a little better through formula and experience alike.
Vinyltriethoxysilane isn’t just another compound on a shelf. Its sharp, solvent-like smell tells you right away that you’re dealing with something that calls for careful handling. Over the years, I’ve watched teams cut corners, storing sensitive chemicals in areas without proper containment. The trouble comes fast: leaky caps, careless stacking, and within weeks, you might smell it even outside the storage area.
Accidents involving silanes almost always trace back to one thing: poor storage. They’re known for reacting with water, even moisture in the air, leading to dangerous buildups of flammable ethanol gas. This isn’t just a checklist issue for a safety manager—it’s a practical, daily concern for anyone anywhere near the warehouse or lab.
Heat invites trouble for Vinyltriethoxysilane. Excess warmth speeds up breakdown, releasing vapors and increasing pressure inside containers, which sometimes split or burst. In my own time overseeing chemical inventories, I saw that the best-run facilities kept thermal spikes in check, installing basic ventilated coolers and keeping chemicals far from direct sunlight or any machinery that kicks out heat.
Moisture makes the situation worse fast. Even if you can barely feel the damp in a room, Vinyltriethoxysilane will find it. Water leaks, roof drips, or even poor air circulation can turn a simple drum into a safety incident waiting to happen. Desiccants might look like overkill, but seasoned workers always rely on them for a reason—they work. Opening a drum should never produce a rush of pressure or a burn in your nostrils.
Quality makes all the difference with containers. I’ve seen companies switch to bargain barrels, thinking they’ll save a few dollars, only to lose thousands replacing product tainted by rust or plastic leaching. Stick to airtight, corrosion-resistant drums—polyethylene works but needs checked regularly for warping or dullness. Lids must seal fully, and gaskets can’t have even the tiniest crack.
Once a container opens, the game changes. Air seeps in, and degradation starts almost immediately. The pros rotate stock using a strict first-in, first-out rule, and label everything with open dates. Little things like wiping off residue and closing containers tightly pay off big.
You can throw out all the guidelines in the world, but unless staff actually buy in, nothing sticks. Having worked both in labs and storerooms, I’ve seen people cut corners only because “someone else will fix it.” Real, hands-on training makes a difference—think mock spills, fire drills, even watching how to screw down a cap properly after pouring.
Digital tools help track inventory, but nothing replaces eyes-on, walk-through inspections. Some companies bring in outside auditors yearly, and it pays off, revealing hidden leaks or slow creep of humidity.
Regulations change. So does Vinyltriethoxysilane’s safety data as manufacturers learn more about its risks and best practices. Refusing to keep up means putting everyone in harm’s way. Industry groups, safety consultants, and regular staff meetings keep everyone focused and informed.
Storing chemicals safely isn’t just for compliance—it’s about protecting people’s lives, company finances, and community trust. Discipline and real engagement are what keep everyone safe, not just a line in a manual.
Vinyltriethoxysilane shows up in more places than most folks realize. Companies rely on this substance to help bond materials, especially in heavy industry, coatings, and adhesives. At the same time, it brings along some real health and safety concerns. That sharp, almost sweet odor hints at something you don’t want to breathe in all day. Inhaling vapors or letting drops linger on skin can lead to headaches, nausea, or irritation. I've seen what happens when folks work around these chemicals without respect—burns, watery eyes, and more than one trip to the health station.
People always talk about personal protective equipment, but being around vinyltriethoxysilane turns talk into action. The usual gloves and goggles don’t cut it. Butyl rubber or nitrile gloves offer solid resistance; those cotton or thin latex gloves will soak through in minutes. A tight-fitting splash goggle—nothing loose or foggy—shields the eyes from surprise sprays. A long-sleeved shirt and pants keep the splashes off skin, with a chemical-resistant apron in the mix for bigger jobs.
Most mishaps happen in closed, stuffy places. Vinyltriethoxysilane vapor creeps through the air, especially on warm days. Throw open the windows, flip on the exhaust, or set up a fume hood. A regular box fan at the door just moves toxic air around—it doesn’t get rid of it. I’ve watched labs invest in solid local exhaust systems after one too many employees complained about dizziness. Now, those same folks barely get a whiff, even on heavy blend days.
Emergencies catch people off guard, but you can't afford to freeze. If it gets on skin, a running sink and soap—use both—will pull it off right away. Eyes need a gentle flush for a quarter of an hour or more. If someone swallows it or inhales too much, getting out in the open air takes precedence over waiting for medical help to arrive. Posting emergency contact numbers and first aid guides in every room with chemicals isn’t just good practice, it genuinely cuts down on panic and lasting harm.
Drums and bottles should never collect dust in the sun or near heaters. Heat degrades vinyltriethoxysilane and sometimes bursts seals. I always push for cool, dry corners—away from strong bases, acids, or moisture. Tightly sealed lids make all the difference. Absorbent pads underneath might save you from cleaning up an expensive mess down the line.
Investing in solid training pays off fast. Newer coworkers often ignore small spills or complain about “one more safety meeting.” Yet those drills stick with you—how to handle a spill kit, recognize warning signs, swap out defective gloves, and file a report. Management needs to walk around, not just leave it to checklists. Fresh eyes spot broken seals, foggy goggles, or stacked drums teetering just wrong.
Real-world safety comes from treating vinyltriethoxysilane with care and respect, not just following paperwork. Good habits protect your skin, lungs, coworkers, and bottom line.
Over the years, plenty of manufacturers and chemists have explored new ways to push materials further. One area where things get interesting is the use of silane coupling agents. Vinyltriethoxysilane pops up a lot in this conversation. It’s not unusual to find projects where a mix of different silanes is on the table, but there’s often some confusion about how these products interact.
It’s easy to get caught up in product names and data sheets, but a closer look at the chemistry always pays off. Vinyltriethoxysilane brings a reactive vinyl group and three ethoxy groups to the mix. It offers a bridge between inorganic and organic phases. This comes in handy for composite materials, coatings, and adhesives. Plenty of applications call for a combination of properties—strong mechanical strength, moisture resistance, or specific adhesion.
Other silane coupling agents carry different groups, like amino, mercapto, or epoxy. Each tweaks the finished product’s properties in its own way. People often want to mix these to cover more bases, but this requires real chemical compatibility, not just a shotgun approach.
Vinyltriethoxysilane tends to play well with others in many systems. The trouble shows up once the hydrolysis and condensation reactions come into play. Vinyl, amino, or epoxy groups all move at their own pace during hydrolysis. If the conditions aren’t just right, you end up with uneven performance. I've seen this mix-and-match approach give unpredictable results in lab trials. Sometimes, one silane dominates the reaction, blocking out the benefits of the others you’ve added.
In silica-filled rubber, for example, using both vinyl and amino silanes can help when you want reactivity with the polymer backbone plus a boost in filler bonding. The trick is matching the ratios, pH level, and processing sequence. Overdosing the vinyl compound can stir up early gelation, killing off any gain from a secondary silane.
Industry data shows that some manufacturers manage to blend Vinyltriethoxysilane with other silanes for coatings and polymer composites. A published study in the Journal of Applied Polymer Science showed gains in tensile strength with dual-silane systems—but only after careful trial-and-error to nail down concentrations and curing conditions. Dow, Momentive, and Shin-Etsu all publish technical guides with warnings about cross-reactivity and the need for careful dosing.
Before diving in, map out what you want from the final product. Sometimes a single well-chosen silane does the trick. In other cases, running small-batch pilot trials helps identify the right ratios and processing settings. Many labs use real-time spectroscopic monitoring to track hydrolysis and surface reactions. Adjusting pH, temperature, or the water-to-silane ratio can save a batch from going sideways.
If in doubt, talk to feedstock suppliers and dig into published case studies. Shared real-world experience often shines a light on the finer points the product sheets skip.
In the end, Vinyltriethoxysilane holds its own with other silane agents, but only in systems where the chemistry lines up with processing. Attention to detail—not just bold mixing—brings out the best in any silane combination.
| Names | |
| Preferred IUPAC name | Triethoxy(ethenyl)silane |
| Other names |
Triethoxyvinylsilane Vinyltriethoxysilane Silane, triethoxyvinyl- Vinylsilane triethoxy- Triethoxy(vinyl)silane |
| Pronunciation | /ˌvaɪ.nəl.traɪˌɛθ.ɒk.siˈleɪn/ |
| Identifiers | |
| CAS Number | 78-08-0 |
| Beilstein Reference | 1361092 |
| ChEBI | CHEBI:87154 |
| ChEMBL | CHEMBL1377702 |
| ChemSpider | 65221 |
| DrugBank | DB14674 |
| ECHA InfoCard | ECHA InfoCard: 100.011.786 |
| EC Number | 213-934-0 |
| Gmelin Reference | 82115 |
| KEGG | C06442 |
| MeSH | C014447 |
| PubChem CID | 15290 |
| RTECS number | VV9275000 |
| UNII | RNF7R1ET66 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID7020173 |
| Properties | |
| Chemical formula | C8H18O3Si |
| Molar mass | 190.32 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 0.89 g/mL at 25 °C (lit.) |
| Solubility in water | Insoluble |
| log P | 0.9 |
| Vapor pressure | 0.5 hPa (20 °C) |
| Acidity (pKa) | 14.7 |
| Basicity (pKb) | 11.5 |
| Magnetic susceptibility (χ) | -7.0E-6 cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 1 – 5 mPa·s |
| Dipole moment | 4.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 431.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -514 kJ/mol |
| Pharmacology | |
| ATC code | V03AC04 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | Flame, Exclamation Mark |
| Signal word | Warning |
| Hazard statements | H226, H319, H332 |
| Precautionary statements | Precautionary statements for Vinyltriethoxysilane are: "P210, P261, P280, P305+P351+P338, P337+P313, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 62 °C |
| Autoignition temperature | 220°C (428°F) |
| Explosive limits | Explosive limits: 1.3–23.3% |
| Lethal dose or concentration | LD50 Oral Rat 10,734 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,460 mg/kg |
| NIOSH | GV7700000 |
| REL (Recommended) | 50 - 200 ppm |
| Related compounds | |
| Related compounds |
Vinyltrimethoxysilane Vinyltris(methoxyethoxy)silane Vinyltriacetoxysilane |